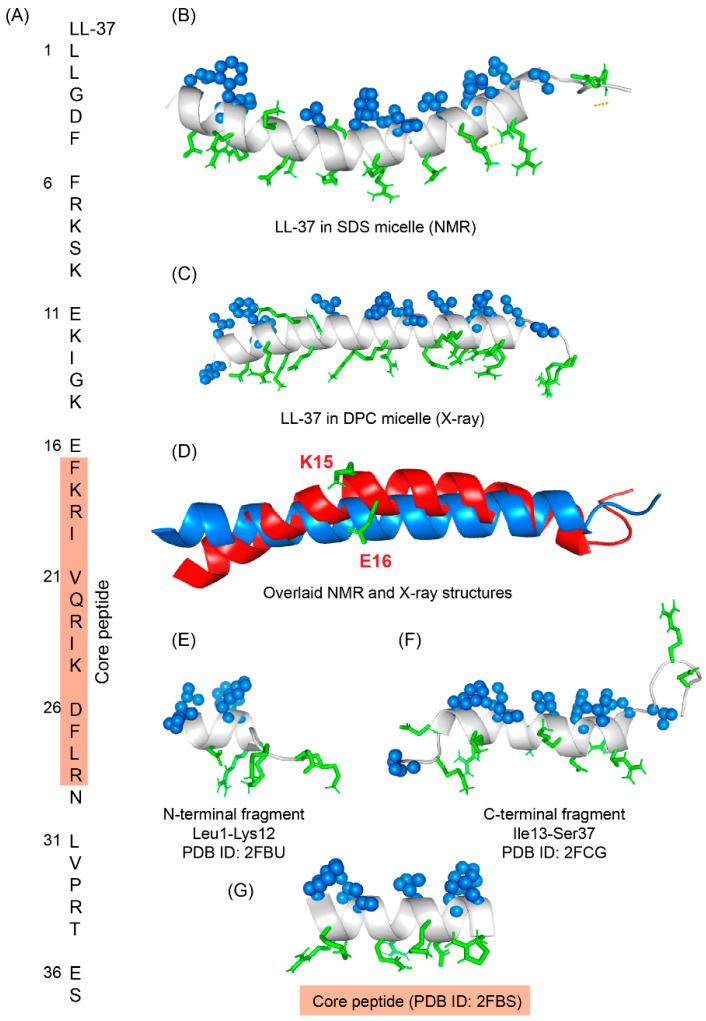
Figure 6.
(A) Amino acid sequence of LL-37 peptide; core peptide is highlighted by maroon-colored box. (B) LL37 adopted a twisted amphipathic α-helical conformation in presence of SDS micelle (PDB: 2K6O) [134]. (C) In presence of DPC micelle, the peptide obtained a straight helical conformation as seen in the X-ray crystallographic analysis (PDB: 5NMN) [135]. (D) The overlaid pdb of NMR and crystallographic structures showed that the SDS bound LL-37 has a kink at residues Lys15 and Glu16. (E–G) The N-terminal, C-terminal, and core peptide fragments also obtained an amphipathic conformation in presence of SDS micelle [136].