Figure 1. Real-world Use of Pan–Fibroblast Growth Factor Receptor (FGFR) Testing and Rate of Erdafitinib Uptake.
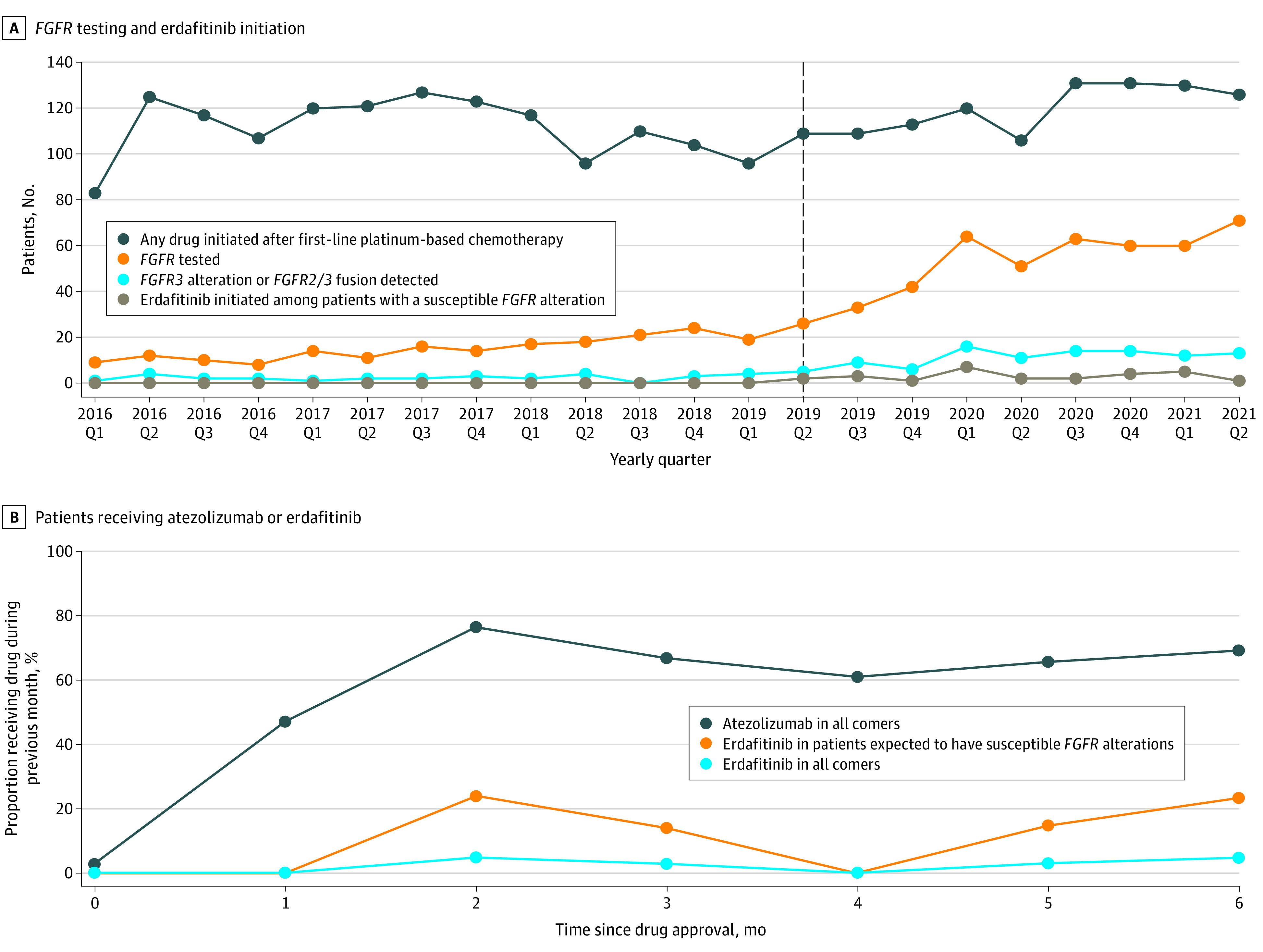
A, The dashed vertical line denotes the timing of approval of erdafitinib. B, The proportions of patients starting a later line of therapy who received atezolizumab and erdafitinib in the 6 months after each drug’s approval (April 12, 2019, to October 8, 2019 for erdafitinib and May 18, 2016, to November 13, 2016 for atezolizumab) are shown. The proportion of patients receiving erdafitinib in the population expected to have a susceptible FGFR alteration was calculated by dividing the proportion of patients starting erdafitinib among all comers by the literature-reported incidence of susceptible FGFR alterations (20%).1 Monthly uptake rates were determined using a linear probability model with robust SEs.
