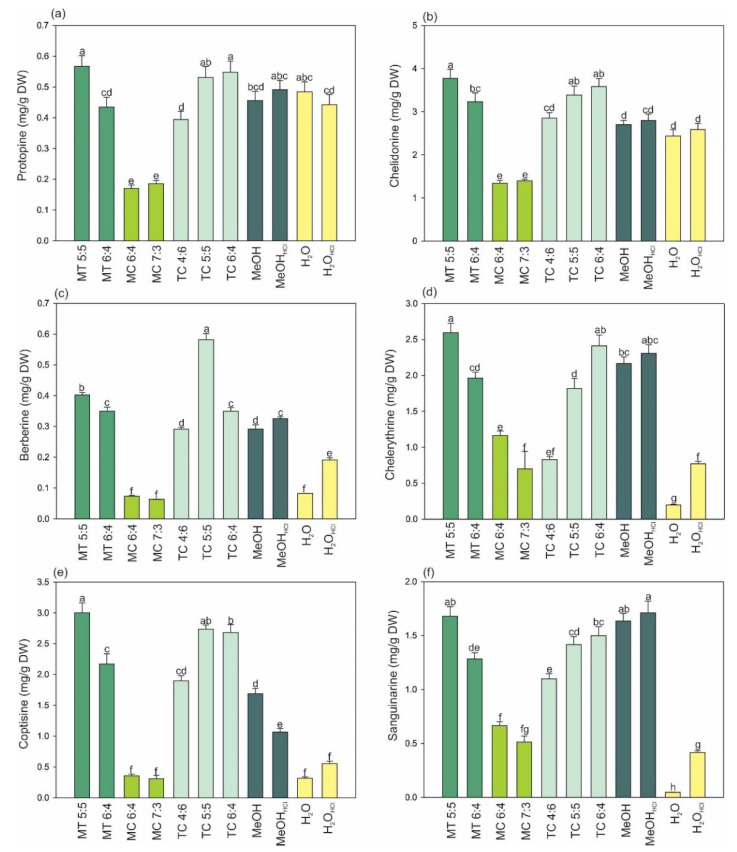
Figure 2.
Yields of extraction of isoquinoline alkaloids from Chelidonium majus roots with volatile natural deep eutectic solvents and commonly used extractants; (a–f), yields of extraction: protopine, chelidonine, berberine, chelerythrine, coptisine, and sanguinarine respectively. MT—Menthol–thymol mixtures; MC—Menthol-camphor mixtures; TC—Thymol-camphor mixtures. The mass:mass ratio of the components in the mixtures is shown next to the symbols. MeOHHCl and H2OHCl-MeOH and water acidified with hydrochloric acid to a concentration of 0.05 M. Data are mean ± SE (n = 5); values for individual raw materials followed by the same letter are not significantly different (p < 0.05, Tukey’s test).