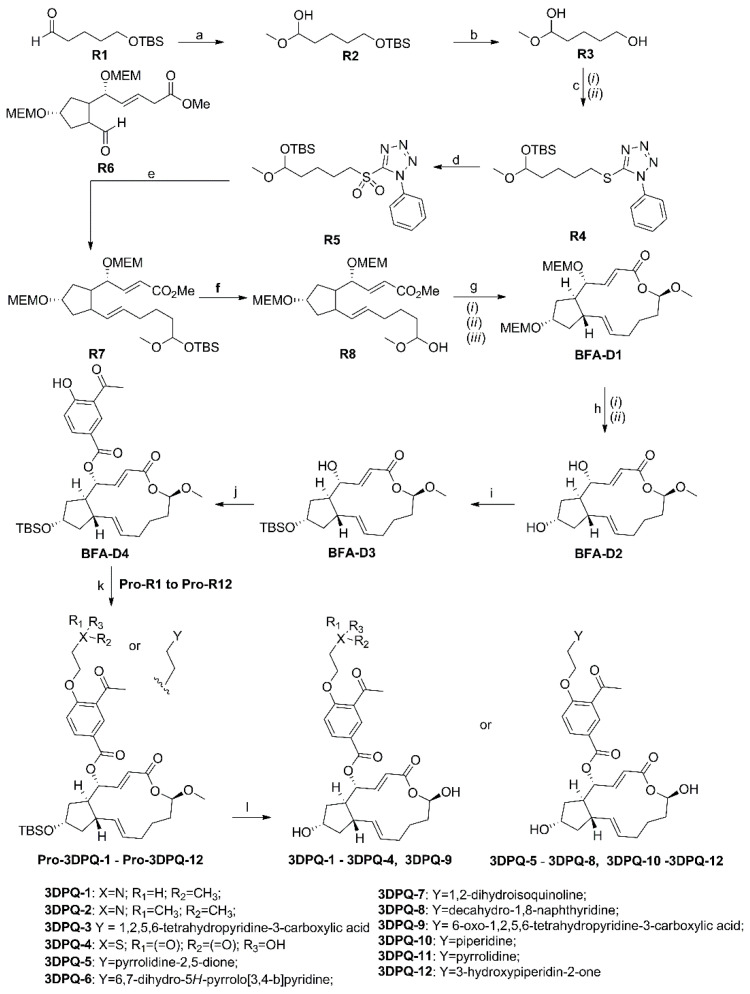
Scheme 1.
Synthesis of Brefeldin A derivatives 3DPQ-1 to 3DPQ-12. Reagents and conditions: (a) Me2Zn, (−)-DBNE, toluene, 0 °C, 24 h, 87% ee; (b) HCl, THF, rt, 25 min; (c) (i) TBS-Cl, imidazole, DMAP, CH2Cl2, 0 °C, 3 h, (ii) PPh3, DEAD, 1-phenyl-1H-tetrazole-5-thiol, THF, 0 °C, 16h; (d) (NH4)6Mo7O24, H2O2, EtOH, rt, 16 h; (e) compound R6, KHDMS, 1,2-dimetoxyethane; -78 °C, 18h; (f) HCl, THF, rt, 1.5 h; (g) (i) LiOH, THF/H2O, rt, 2h, (ii) 2,4,6-trichlobenzoylchloride, NEt3, THF, rt, 1.5 h, (iii) DMAP, toluene, reflux, 5h; (h) (i) cc HBR, THF, rt, 1.5 h (ii) recrystallization; (i) TBSOTf, 2,6-lutidine, CH2Cl2, rt; (j) 3-acetyl-4-hydroxybenzoic acid, ECD, DMAP, CH2Cl2, reflux; (k) K2CO3, EtOH, reflux; (l) (i) TBAF, THF, rt, (ii) BBr3, CH2Cl2, 0 °C, 3h, reflux.