Fig. 3.
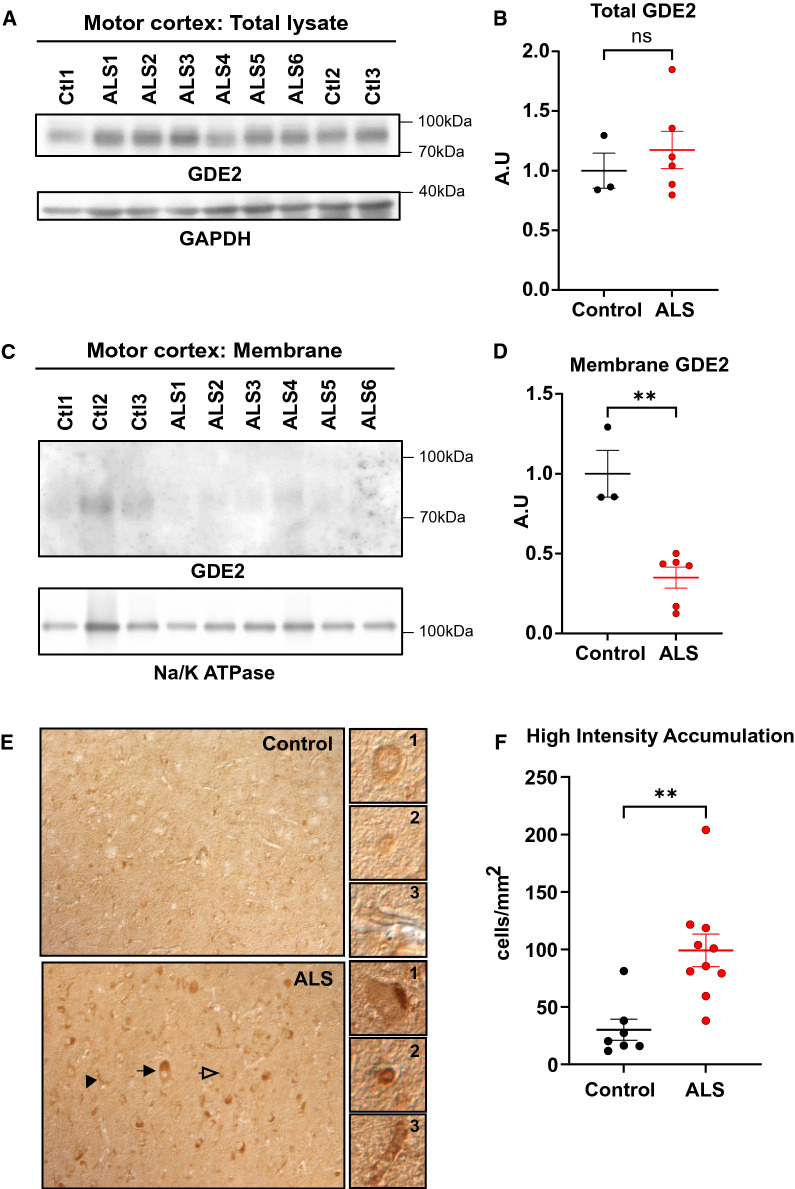
hGDE2 protein distribution is disrupted in ALS. A Western blot of protein extracts prepared from postmortem motor cortex of control (Ctl) and patients with ALS. GAPDH serves as a loading control. B Quantification of hGDE2 expression relative to GAPDH in total lysate shows no significant (ns) difference in hGDE2 expression between control and ALS patients. Graph represents mean ± SEM. Student’s t test (p = 0.6985), n = 3 Control, 6 ALS. C Western blot of Triton-X-114 membrane fractions of motor cortex from control individuals and patients with ALS. Na/K ATPase serves as a loading control and confirms the enrichment of membrane proteins in extracts. D Quantification of hGDE2 expression relative to Na/K ATPase in membrane extracts shows reduced hGDE2 expression in membrane fractions from ALS patients compared to control individuals. Graph represents mean ± SEM. Student’s t test (**p = 0.0020), n = 3 Control, 6 ALS. E Immunohistochemical staining of postmortem human motor cortex sections of control individuals and patients with ALS. Arrows highlight different types of cells with high-intensity hGDE2 accumulations (black arrow = neuron, clear arrow = glial cell, black arrowhead = blood vessel). Representative images of GDE2 accumulations in these different types of cells compared with control are highlighted in panels 1 (neuron), 2 (glial cell), and 3 (blood vessel). F Quantification of the number of high-intensity hGDE2 accumulations in cells from control and ALS sections shows a significant increase of high intensity accumulations of hGDE2 in ALS patients compared to controls. Total number of cells counted: Control = 831 and ALS = 2896. Graph represents mean ± SEM. Student’s t test (**p = 0.0022), n = 7 Control, 10 ALS