Abstract
Background
An aberrant expression of long non‐coding RNA PVT1 has been associated with apoptosis in various cancer types. We aimed to explore the PVT1 and four apoptosis‐related proteins (p53, Bcl2, and PD‐1/PD‐L1) signature in thyroid cancer (TC).
Methods
The PVT1 expression level was measured in 64 FFPE TC paired samples by real‐time quantitative PCR. Overall and stratified analyses by different clinicopathological features were done. The apoptotic proteins were evaluated by immunohistochemistry staining.
Results
Overall analysis showed significant PVT1upregulation in TC tissues (p < 0.001). Similarly, subgroup analysis by BRAF V600E mutation showed consistent results. Lower expression of p53 was associated with mortality (p = 0.001). Bcl2 overexpression was associated with greater tumor size (p = 0.005). At the same time, HCV‐positive cases were associated with repressed Bcl2 expression levels (54.3% in HCV‐negative vs. 6.9% in HCV‐positive cases, p = 0.011). PD‐1 expression was associated with lymph node metastasis (p = 0.004). Enhanced PD‐L1 expression in the tumor was associated with a higher tumor stage, lymphovascular invasion, and mortality risk. Kaplan–Meier curves for overall survival showed that low p53 and high PD‐L1 expressions were associated with lower survival time. The p53‐positive staining is associated with a 90% decreased mortality risk (HR = 0.10, 95%CI = 0.02–0.47, p = 0.001), while patients with high PD‐L1 were five times more likely to die (HR = 4.74, 95%CI = 1.2–18.7, p = 0.027).
Conclusion
Our results confirm the upregulation of PVT1 in TC. The apoptosis‐related proteins (p53, Bcl2, and PD‐1/PD‐L1) showed different prognostic utility in TC patients; in particular, low p53 and high PD‐L1 expressions associated with low survival times. Further large‐scale and mechanistic studies are warranted.
Keywords: Bcl2, immunohistochemistry, P53, papillary thyroid cancer, PD‐1, PD‐L1, PVT1, real‐time PCR
The workflow of explored lncRNA PVT1 and apoptosis‐related proteins in thyroid cancer
1. INTRODUCTION
Thyroid cancer (TC) is the most common endocrine tumor worldwide. 1 Understanding the pathogenesis of TC and finding biomarkers for its early diagnosis and effective treatment are current focal points of research. 2 , 3 , 4 Accumulating evidence indicates that several protein‐coding and non‐coding genes play essential roles in TC development and progression. 2 , 5 , 6 , 7 , 8
Long non‐coding RNAs (lncRNA), a group of endogenous cellular transcripts more than 200 nucleotides in length, have been involved in multiple pathophysiological processes of the human body, especially tumorigenesis and progression of cancers. 9 The aberrant expression of lncRNA processes crucial functions involved in proliferation, apoptosis, and metastasis through abnormal regulation of gene expression transcriptionally and post‐transcriptionally. 10 Given the high tissue specificity, efficiency, and stability, lncRNAs might be used as biomarkers for diagnosis/prognosis or monitoring of human cancers. 11
In the last decade, the lncRNA plasmacytoma variant translocation 1 (PVT1) has gained significant attention due to being verified to mediate tumorigenesis in multiple cancers. PVT1 gene, also named as MIR1204HG, MYC Activator, LINC00079, and Onco‐LncRNA‐100, is located on chromosome 8q24.21, 100 to 500 kb 3‐prime of MYC, hosting four microRNAs gene cluster, namely MIR1204, 1205, 1206, 1207, and 1208, and can have 176 splice variant transcripts of varying length (https://www.ncbi.nlm.nih.gov/gene/5820). Deregulated expression of PVT1 has been demonstrated to be associated with solid organ 12 , 13 , 14 and hematological malignancies, 15 and its deregulation could be related to survival and prognosis of cancer patients. 16 , 17 , 18 Pertinent to clinical practice, PVT1 might act as a prognostic biomarker for tumors and serve as a potential target for therapy. 19
In some tumors, PVT1 was related to apoptosis, as evident in colorectal cancer 16 and malignant pleural mesothelioma. 20 Tetracycline‐inducible shRNA targeting PVT1 inhibited cell growth and induced apoptosis in bladder cancer cells. 21 Similarly, PVT1 knockdown affected proliferation and promoted apoptosis of uveal melanoma cells by inhibiting the "enhancer of zeste homolog 2 (EZH2)" protein. 22 In lung squamous cell carcinoma, knockdown of PVT1 inhibited LUSC cell growth. 23 However, the underlying molecular role of PVT1 in thyroid cancer (TC) is still in its infancy.
Previous studies identified the potential mutual relation of PVT1 with some apoptosis‐related coding genes expression in cancer tissues, including the p53, 24 , 25 Bcl2, 26 and PD‐1/PD‐L1. 27 Given that PVT1 represents a promising novel biomarker for various cancer types and has a great potential to be effectively used in clinical practice in the near future, exploring its role in thyroid cancer will add to the knowledge. Here, we aimed to identify the relative expression of PVT1 and four apoptosis‐related proteins (p53, Bcl2, PD‐1, and PD‐L1) expression signature in thyroid cancer compared to paired non‐cancer tissues using real‐time PCR and immunohistochemistry, respectively. Furthermore, the association of this signature with different clinicopathological parameters of TC patients was investigated.
2. SUBJECTS AND METHODS
2.1. Specimen collection
A total of 128 thyroid specimens (64 cancer tissues and their paired non‐cancer adjacent tissues) were included in the current analysis. Formalin‐fixed paraffin‐embedded (FFPE) archival samples were retrieved from Mansoura and Suez Canal University Pathology Labs, Egypt. The "Declaration of Helsinki" guidelines were followed in this work. Ethical approval was provided by the Medical Research Ethics Committee, Faculty of Medicine, Suez Canal University, Egypt. Patient information was collected and managed using anonymous codes. Patients were 18–60 years old and underwent thyroidectomy and/or lobectomy. Patients with the following criteria were excluded: under 18 years old, with non‐papillary TC, secondary carcinoma, missing clinical data, loss of follow‐up, unmatched cohorts, no available archiva paraffin blocks, and/or blocks with insufficient tissue to do complete immunohistochemical staining.
2.2. Histopathological analysis
A histopathological expert assessed the specimens to confirm the diagnosis and discriminate between cancer and non‐cancer regions. FFPE blocks were cut into sections of 4‐micron thickness, stained with H&E, examined for tumor diagnosis, and evaluated for other microscopic parameters, such as histological subtype, the staging of cancer, stromal lymphocytes, extra‐thyroid extension, lymph node metastasis, lymphovascular and perineural invasion. The TNM (tumor, node, and metastasis) classification of the "American Joint Committee on Cancer (AJCC, 8th edition)" was applied for tumor staging". 28
2.3. Clinical assessment and follow‐up
Patient information was obtained from medical records. They included patients’ demographic data, primary cancer site, pathology reports, and treatment modalities (surgery, radioactive iodine therapy, and thyroxin intake). Post‐thyroidectomy, patients have monitored every six months in which they underwent clinical examination, imaging studies, and serum thyroglobulin level estimation. Relapse, recurrence, and death were reported at follow‐up.
2.4. Gene expression analysis
Qiagen RNeasy FFPE Kit (Cat. #73504, Qiagen, Hilden, Germany) was used to extract total RNA according to the manufacturer's instructions. 29 Quantity and purity of extracted RNA were determined using NanoDrop ND‐1000 spectrophotometer (NanoDrop Tech., Inc.). As previously described, complementary DNA (cDNA) was prepared using High‐capacity RNA‐to‐cDNA Synthesis Kit (Cat. #4390779). 30 Real‐time quantitative polymerase chain reaction (PCR) was followed using Universal Master Mix (Cat. #4440042) and TaqMan assay for PVT1 (Assay ID: Hs00413039_m1, Applied Biosystems, Thermo Fisher Scientific Inc.). Reactions were carried out in a T‐Professional Basic, Biometra PCR System (Biometra, Goettingen, Germany) for thermocycler and StepOne™ Real‐Time PCR System (Applied Biosystems) for real‐time PCR following the supplied manufacturer's instructions. The housekeeping gene "glyceraldehyde 3‐phosphate dehydrogenase (GAPDH)" was quantified (Assay ID: Hs02786624_g1) in the samples for normalization of the PCR data. Appropriate negative controls were included in each run (no template and no enzyme samples), and duplicate PCR runs were performed. The "Minimum Information for Publication of Quantitative Real‐Time PCR Experiments (MIQE)" guidelines were followed during the experiments. 31 Fold changes of PVT1 gene expression in each patient's cancer tissue relative to the corresponding non‐cancer adjacent tissues (NAT) were estimated via the "Livak method" based on the quantification (threshold) cycle (Cq or CT) value: relative gene expression =2−ΔΔ Cq . 32
2.5. Immunohistochemistry analysis
For BRAF V600E mutation analysis, 5 µm thick sections of FFPE tissues were prepared. VE1 IHC method was applied as detailed previously. 33 Normal thyroid tissues were run as a negative control. For apoptosis‐related protein expression analysis, the deparaffinized tissue sections were pretreated with hydrogen peroxide (3%) solution and incubated at room temperature for 15 min to remove endogenous peroxidase. Antigen retrieval was performed in sodium citrate buffer solution for 5 min using a microwave oven, followed by automatic cooling at room temperature. Then tissues were incubated with primary antibodies as follows: Anti‐p53 antibodies (a rabbit polyclonal antibody IgG (clone YPA2006; Chongqing Biopsies CO., Ltd.) with dilution 1:200 and pH 7.4, Anti‐Bcl2 antibodies (Mouse monoclonal anti‐Bcl2. CELL MARQUE, code No 226 M‐98, 7 ml prediluted, anti‐PD‐L1 (Clone, YPA1638, Biospes, Chongqing Biospes Co., Ltd,), and anti‐PD‐1 (Clone, YPA1637, Biospes, Chongqing Biospes Co., Ltd,). The whole tissue was covered with antibodies and incubated 8 h in a refrigerator at 4°C. For secondary antibody incubation, tissue samples were incubated with the streptavidin‐biotin complex method using a SAB‐PO kit (Nichirei, Tokyo, Japan) at room temperature for one hour. Antigen detection was carried out by placing diaminobenzidine on each section. The slides were counterstained with hematoxylin and dehydrated in alcohol and xylene before mounting slides. For negative controls, sections were treated the same way, but they were incubated with antibody diluent instead of the primary antibody. Positive controls were used parallel (colon cancer for p53; Tonsil tissue for PD‐1 and PD‐L1). The photos were obtained using a Nikon magnifying lens prepared with a 5–megapixel cooled CCD camera joined with the Picture Pro Plus AMS7 computer program.
2.6. Interpretation of immunohistochemical results
An expert pathologist has assessed the immunostained slides blindly to the clinicopathological features. For p53, if more than 25% of tissue were stained, it was considered a p53 positive sample. For Bcl2, it scored negative if ≤5% of neoplastic cells were stained and considered positive if scored from 6% to 100%. The value of Bcl2 was considered low expression if 6 to less than 50% were brown stained and high expression if ≥50% of tumor cells were brown stained. 34
Regarding the positive immunostaining of PD‐1 and PD‐L1 in the tumor cells and immune cells, the intensity of the stain was scored as 0 (no staining), 1 (light yellow), 2 (brown), and 3 (deep brown). The number of stained cells per 100 was scored as 1 (≤10%), 2 (10% ~ 50%), and 3 (≥50%). High PD‐L1 expression was detected when the staining strength score was multiplied, and the number of stained cells per 100 cells was no less than three. Regarding immune cell‐specific PD‐L1 and PD‐1 expression, the percentage of stained cells per 100 cells was detected and categorized as 0%–9%, 10%–49%, and 50–100% stained immune cells. Therefore, >50% was used as a cutoff for high expression for PD‐1 and PD‐L1. 35 , 36
2.7. Statistical analysis
Data were managed using the "Statistical Package for the Social Sciences (SPSS) for Windows" software (version 22.0), BioVinci (version 1.1.3), R version 3.5.3, and R studio version 1.1.383. Categorical variables were compared using the chi‐square (χ2) or Fisher's exact tests where appropriate, while the student's t‐test or Mann‐Whitney U tests were used to compare continuous variables. Log Rank (Mantel‐Cox) test was used to estimate the overall survival (OS) time defined as "the interval between the time of surgery to death or the last follow‐up," and the disease‐free survival (DFS) time defined as "the time length that the patient survives after primary cancer treatment ends without any signs/symptoms of that cancer" of TC patients. Meanwhile, Kaplan–Meier survival curves were generated for the OS and DFS. Multivariate Cox regression analysis was applied to identify predictors for mortality. A two‐tailed p‐value of <0.05 was considered significant.
3. RESULTS
3.1. Characteristics of the study population
A total of 64 patients with up to a 7‐years follow‐up period were included in the current study. Of these, 70.3% were younger than 55 years old, 78.1% were females, and 43.7% were BRAF mutant. There were no significant clinical or pathological differences between patients with BRAF mutant and BRAF wild types (Table 1).
TABLE 1.
Characteristics of study population according to BRAF V600E mutation
| Patient Characteristics | Levels | Total (N = 64) | BRAF wild type (N = 36) | BRAF mutant (N = 28) | p‐value |
|---|---|---|---|---|---|
| Demographic data | |||||
| Age, years | <55 y | 45 (70.3) | 25 (69.4) | 20 (71.4) | 0.86 |
| ≥55 y | 19 (29.7) | 11 (30.6) | 8 (28.6) | ||
| Sex | Male | 14 (21.9) | 7 (19.4) | 7 (25) | 0.76 |
| Female | 50 (78.1) | 29 (80.6) | 21 (75) | ||
| Pathological assessment | |||||
| Laterality | Unilateral | 47 (73.4) | 26 (72.2) | 21 (75) | 0.80 |
| Bilateral | 17 (26.6) | 10 (27.8) | 7 (25) | ||
| Histological variant | Classical | 32 (50) | 21 (58.3) | 11 (39.3) | 0.16 |
| Follicular | 24 (37.5) | 13 (36.1) | 11 (39.3) | ||
| Oncocytic | 2 (3.1) | 0 (0) | 2 (7.1) | ||
| Tall cell | 6 (9.4) | 2 (5.6) | 4 (14.3) | ||
| Pathology Stage | Stage I | 48 (75) | 28 (77.8) | 20 (71.4) | 0.72 |
| Stage II | 5 (7.8) | 3 (8.3) | 2 (7.1) | ||
| Stage IVB | 11 (17.2) | 5 (13.9) | 6 (21.4) | ||
| T stage | T1 | 15 (23.4) | 7 (19.4) | 8 (28.6) | 0.41 |
| T2 | 24 (37.5) | 16 (44.4) | 8 (28.6) | ||
| T3 | 25 (39.1) | 13 (36.1) | 12 (42.9) | ||
| N stage | N0 | 29 (45.3) | 17 (47.2) | 12 (42.9) | 0.92 |
| N1a | 16 (25) | 9 (25) | 7 (25) | ||
| N1b | 19 (29.7) | 10 (27.8) | 9 (32.1) | ||
| M stage | M0 | 53 (82.8) | 31 (86.1) | 22 (78.6) | 0.51 |
| M1 | 11 (17.2) | 5 (13.9) | 6 (21.4) | ||
| Focality | Unifocal | 40 (62.5) | 20 (55.6) | 20 (71.4) | 0.29 |
| Multifocal | 24 (37.5) | 16 (44.4) | 8 (28.6) | ||
| Extrathyroidal extension | Negative | 53 (82.8) | 31 (86.1) | 22 (78.6) | 0.51 |
| Positive | 11 (17.2) | 5 (13.9) | 6 (21.4) | ||
| Lymphovascular invasion | Negative | 55 (85.9) | 32 (88.9) | 23 (82.1) | 0.48 |
| Positive | 9 (14.1) | 4 (11.1) | 5 (17.9) | ||
| Perineural invasion | Negative | 62 (96.9) | 35 (97.2) | 27 (96.4) | 0.85 |
| Positive | 2 (3.1) | 1 (2.8) | 1 (3.6) | ||
| Lymphocyte enrichment | Negative | 33 (51.6) | 16 (44.4) | 17 (60.7) | 0.21 |
| Positive | 31 (48.4) | 20 (55.6) | 11 (39.3) | ||
| HCV antibody | Negative | 43 (67.2) | 27 (75) | 16 (57.1) | 0.18 |
| Positive | 21 (32.8) | 9 (25) | 12 (42.9) | ||
| Intervention | |||||
| Thyroidectomy | Unilateral | 9 (14.1) | 4 (11.1) | 5 (17.9) | 0.48 |
| Total/subtotal | 55 (85.9) | 32 (88.9) | 23 (82.1) | ||
| Neck dissection | Negative | 18 (28.1) | 10 (27.8) | 8 (28.6) | 0.94 |
| Positive | 46 (71.9) | 26 (72.2) | 20 (71.4) | ||
| Residual after resection | Negative | 37 (57.8) | 19 (52.8) | 18 (64.3) | 0.44 |
| Positive | 27 (42.2) | 17 (47.2) | 10 (35.7) | ||
| Received Eltroxin | Negative | 30 (46.9) | 15 (41.7) | 15 (53.6) | 0.45 |
| Positive | 34 (53.1) | 21 (58.3) | 13 (46.4) | ||
| RAI | Negative | 36 (56.3) | 17 (47.2) | 19 (67.9) | 0.13 |
| Positive | 28 (43.8) | 19 (52.8) | 9 (32.1) | ||
| EBRT | Negative | 59 (92.2) | 35 (97.2) | 24 (85.7) | 0.15 |
| Positive | 5 (7.8) | 1 (2.8) | 4 (14.3) | ||
| Follow‐up | |||||
| Progression | Negative | 35 (54.7) | 18 (50) | 17 (60.7) | 0.45 |
| Positive | 29 (45.3) | 18 (50) | 11 (39.3) | ||
| Mortality | Survived | 53 (82.8) | 29 (80.6) | 24 (85.7) | 0.74 |
| Died | 11 (17.2) | 7 (19.4) | 4 (14.3) | ||
Data are represented as frequency (percentage).
Abbreviations: RAI: Radioactive iodine, EBRT: External beam radiotherapy, Progression: included recurrence, relapse, and distant metastasis. A two‐sided Chi‐square test was applied. Statistical significance was set at p‐value <0.05.
3.2. PVT1 gene expression level
The overall analysis of 64 paired tissues showed upregulation of the PVT1 gene in cancer tissues compared to paired adjacent non‐cancer tissues (p < 0.001). Similarly, subgroup analysis by BRAF mutation showed consistent results (Figure 1). PVT1 upregulation was observed in 73.8% (N = 48) of cases. Otherwise, PVT1 expression was not associated with clinical or pathological features (Table 2).
FIGURE 1.
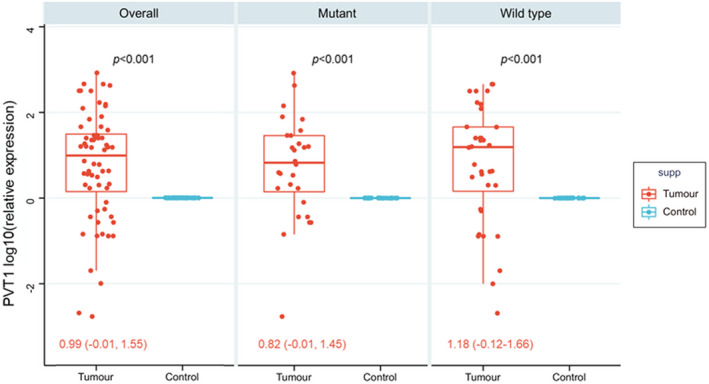
PVT1 gene expression profile in thyroid cancer tissues compared to normal counterparts. Paired t‐test was used to compare cancer and non‐cancer tissues. Overall analysis and stratification by BRAF V600E mutation are shown. All p‐values were <0.001. Fold change was normalized by the GAPDH housekeeping gene. The median and inteq1uartile fold change range of tumor specimens relative to non‐cancer paired tissues are shown
TABLE 2.
Association of PVT1 gene expression and clinicopathological characteristics
| Characteristics | Levels | Low expression | High expression | p‐value |
|---|---|---|---|---|
| Demographic data | ||||
| Age, years | <55 year | 13 (81.3) | 32 (66.7) | 0.35 |
| ≥55 year | 3 (18.8) | 16 (33.3) | ||
| Sex | Male | 2 (12.5) | 12 (25) | 0.48 |
| Female | 14 (87.5) | 36 (75) | ||
| Pathological assessment | ||||
| Laterality | Unilateral | 12 (75) | 35 (72.9) | 0.87 |
| Bilateral | 4 (25) | 13 (27.1) | ||
| Histological variant | Classical | 8 (50) | 24 (50) | 0.77 |
| Follicular | 7 (43.8) | 17 (35.4) | ||
| Oncocytic | 0 (0) | 2 (4.2) | ||
| Tall cell | 1 (6.3) | 5 (10.4) | ||
| Pathology Stage | Stage I/II | 15 (93.8) | 38 (79.2) | 0.83 |
| Stage IVB | 1 (6.3) | 10 (20.8) | ||
| Tumor size | T1/2 | 9 (56.3) | 30 (62.5) | 0.48 |
| T3 | 7 (43.8) | 18 (37.5) | ||
| LN metastasis | Positive | 8 (50) | 27 (56.3) | 0.79 |
| Distant metastasis | Positive | 1 (6.3) | 10 (20.8) | 0.26 |
| Focality | Multifocal | 4 (25) | 20 (41.7) | 0.37 |
| ETE | Positive | 2 (12.5) | 9 (18.8) | 0.71 |
| LVI | Positive | 4 (25) | 5 (10.4) | 0.21 |
| Lymphocyte enrichment | Positive | 8 (50) | 23 (47.9) | 0.88 |
| BRAF V600E | Mutant | 7 (43.8) | 21 (43.8) | 0.99 |
| HCV antibody | Positive | 2 (12.5) | 19 (39.6) | 0.06 |
| Follow‐up | ||||
| Progression | Positive | 6 (37.5) | 23 (47.9) | 0.56 |
| Mortality | Died | 2 (12.5) | 9 (18.8) | 0.71 |
Data are represented as frequency (percentage).
Abbreviations: ETE: Extrathyroidal extension; LN: lymph node; LVI: Lymphovascular invasion. Progression: included recurrence, relapse, and distant metastasis. A two‐sided Chi‐square test was used. Statistical significance was set at p‐value <0.05.
3.3. Apoptosis‐related protein expression
Immunohistochemistry reactivity is shown in Figures 2 and 3. Positive staining of p53 was observed in 51 cases (79.6%). Bcl2 protein expression was found in 35 samples (54.6%). At the same time, PD‐1 and PD‐L1 were positively stained in 52 (81.3%) and 18 (28.1%) patients, respectively (Figure S1). As depicted in Table 3, females were more likely to have higher p53 protein staining than males (84.3% vs. 15.7%, p = 0.030). Lower expression of p53 was associated with mortality (53.8% vs. 7.8% in high expressors, p = 0.001). Protein expression of the anti‐apoptotic Bcl2 varied across histopathological variants (p = 0.012). Overexpression was associated with greater tumor size (58.6% vs. 22.9%, p = 0.005), while HCV‐positive cases were associated with repressed expression levels (54.3% in HCV negative vs. 6.9% in HCV positive, p = 0.011). PD‐1 protein was associated with lymph node metastasis (63.5% vs. 16.7%, p = 0.004). For its ligand, PD‐L1 protein, elders (55.6% vs. 19.6%, p = 0.007) and males (44.4% vs. 13.0%, p = 0.015) were more likely to exhibit higher protein staining. Enhanced PD‐L1 expression in tumor was associated with higher tumor stage (72.2% vs. 26.1%, p = 0.001), lymphovascular invasion (44.4% vs. 2.2%, p < 0.001), and risk of mortality (38.9% vs. 8.7%, p = 0.008).
FIGURE 2.
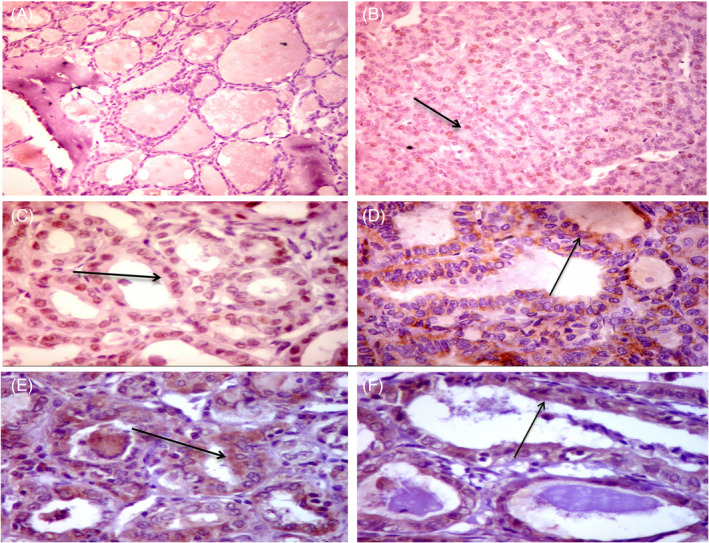
Immunohistochemical (IHC) staining for p53 and BCL2 proteins in papillary thyroid carcinoma (PTC). IHC staining for p53 protein showed negative staining in non‐cancerous thyroid tissue (AX200) and scattered nuclear staining for p53 in PTC (Black arrow) (BX200). Diffuse nuclear staining for p53 in follicular variant of PTC (black arrow) (CX400). Staining for BCL2 protein showed focal mild cytoplasmic staining for BCL2 in hyperplastic nodule (DX 400), diffuse strong cytoplasmic staining for BCL2 in follicular variant of PTC (black arrow) (EX400), and moderate cytoplasmic staining in PTC (FX400)
FIGURE 3.
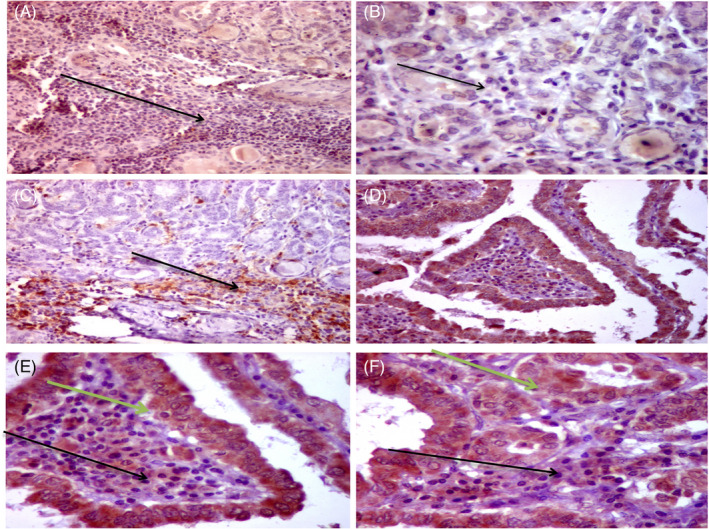
Immunohistochemical (IHC) staining for PD‐1 and PD‐L1 in papillary thyroid carcinoma (PTC). Thyroid papillary carcinoma follicular variant with dense intra‐tumoral immune cells (yellow arrow) showed negative staining of PD‐1 protein in intracellular immune cells (ICCs) of PTC (AX100). Higher power of the same case showed negative staining for PD‐1 in ICCs (black arrow) (BX200). Positive staining of PD‐1 protein in ICCs in follicular variant of PTC (Black arrow) (CX100). Diffuse positive staining for PD‐L1 protein in both tumor cells and intra‐tumoral immune cells in PTC (classic variant) (DX200). Higher power of the same case (D) showed diffuse strong cytoplasmic staining of all tumor cells (green arrows) and most of the ICCs for PD‐L1 (black arrows) (EX400). A follicular variant of PTC showed diffuse positive staining for PD‐L1 in tumor (green arrow) and immune cells (black arrow) (FX400)
TABLE 3.
Association of protein expression levels and clinicopathological features
| Characteristics | Levels | p53 | bcl2 | PD−1 | PD‐L1 (Tumor) | PD‐L1 (lymphocyte) | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Low expression | High expression | Low expression | High expression | Low expression | High expression | Low expression | High expression | Low expression | High expression | ||
| Demographic data | |||||||||||
| Age, years | <55 y | 7 (53.8) | 38 (74.5) | 27 (77.1) | 18 (62.1) | 10 (83.3) | 35 (67.3) | 37 (80.4) | 8 (44.4)** | 23 (69.7) | 22 (71) |
| ≥55 y | 6 (46.2) | 13 (25.5) | 8 (22.9) | 11 (37.9) | 2 (16.7) | 17 (32.7) | 9 (19.6) | 10 (55.6) | 10 (30.3) | 9 (29) | |
| Sex | Male | 6 (46.2) | 8 (15.7)* | 7 (20) | 7 (24.1) | 1 (8.3) | 13 (25) | 6 (13) | 8 (44.4)* | 8 (24.2) | 6 (19.4) |
| Female | 7 (53.8) | 43 (84.3) | 28 (80) | 22 (75.9) | 11 (91.7) | 39 (75) | 40 (87) | 10 (55.6) | 25 (75.8) | 25 (80.6) | |
| Pathological assessment | |||||||||||
| Laterality | Unilateral | 7 (53.8) | 40 (78.4) | 25 (71.4) | 22 (75.9) | 9 (75) | 38 (73.1) | 33 (71.7) | 14 (77.8) | 24 (72.7) | 23 (74.2) |
| Bilateral | 6 (46.2) | 11 (21.6) | 10 (28.6) | 7 (24.1) | 3 (25) | 14 (26.9) | 13 (28.3) | 4 (22.2) | 9 (27.3) | 8 (25.8) | |
| Histological variant | Classical | 8 (61.5) | 24 (47.1) | 22 (62.9) | 10 (34.5) * | 3 (25) | 29 (55.8) | 24 (52.2) | 8 (44.4) | 15 (45.5) | 17 (54.8) |
| Follicular | 3 (23.1) | 21 (41.2) | 7 (20) | 17 (58.6) | 6 (50) | 18 (34.6) | 15 (32.6) | 9 (50) | 15 (45.5) | 9 (29) | |
| Oncocytic | 0 (0) | 2 (3.9) | 1 (2.9) | 1 (3.4) | 1 (8.3) | 1 (1.9) | 2 (4.3) | 0 (0) | 1 (3) | 1 (3.2) | |
| Tall cell | 2 (15.4) | 4 (7.8) | 5 (14.3) | 1 (3.4) | 2 (16.7) | 4 (7.7) | 5 (10.9) | 1 (5.6) | 2 (6.1) | 4 (12.9) | |
| Pathology Stage | Stage I/II | 9 (69.2) | 44 (86.3) | 31 (88.6) | 22 (75.9) | 12 (100) | 41 (78.8) | 41 (89.1) | 12 (66.7) | 24 (72.7) | 29 (93.5) |
| Stage IVB | 4 (30.8) | 7 (13.7) | 4 (11.4) | 7 (24.1) | 0 (0) | 11 (21.2) | 5 (10.9) | 6 (33.3) | 9 (27.3) | 2 (6.5) | |
| Tumor size | T1/2 | 9 (69.2) | 30 (58.8) | 27 (77.1) | 12 (41.4)** | 9 (75) | 30 (57.7) | 34 (73.9) | 5 (27.8)** | 24 (72.7) | 29 (93.5) |
| T3 | 4 (30.8) | 21 (41.2) | 8 (22.9) | 17 (58.6) | 3 (25) | 22 (42.3) | 12 (26.1) | 13 (72.2) | 9 (27.3) | 2 (6.5) | |
| LN metastasis | Positive | 7 (53.8) | 28 (54.9) | 16 (45.7) | 19 (65.5) | 2 (16.7) | 33 (63.5)** | 26 (56.5) | 9 (50) | 17 (51.5) | 18 (58.1) |
| Distant metastasis | Positive | 4 (30.8) | 7 (13.7) | 4 (11.4) | 7 (24.1) | 0 (0) | 11 (21.2) | 5 (10.9) | 6 (33.3) | 9 (27.3) | 2 (6.5) |
| Focality | Multifocal | 8 (61.5) | 16 (31.4) | 14 (40) | 10 (34.5) | 6 (50) | 18 (34.6) | 20 (43.5) | 4 (22.2) | 9 (27.3) | 15 (48.4) |
| ETE | Positive | 3 (23.1) | 8 (15.7) | 6 (17.1) | 5 (17.2) | 0 (0) | 11 (21.2) | 9 (19.6) | 2 (11.1) | 3 (9.1) | 8 (25.8) |
| LVI | Positive | 3 (23.1) | 6 (11.8) | 2 (5.7) | 7 (24.1) | 0 (0) | 9 (17.3) | 1 (2.2) | 8 (44.4)*** | 6 (18.2) | 3 (9.7) |
| BRAFV600E | Mutant | 6 (46.2) | 22 (43.1) | 18 (51.4) | 10 (34.5) | 3 (25) | 25 (48.1) | 20 (43.5) | 8 (44.4) | 17 (51.5) | 11 (35.5) |
| HCV Ab | Positive | 4 (30.8) | 17 (33.3) | 19 (54.3) | 2 (6.9)* | 5 (41.7) | 16 (30.8) | 18 (39.1) | 3 (16.7) | 10 (30.3) | 11 (35.5) |
| Follow‐up | |||||||||||
| Progression | Positive | 7 (53.8) | 22 (43.1) | 17 (48.6) | 12 (41.4) | 6 (50) | 23 (44.2) | 21 (45.7) | 8 (44.4) | 12 (36.4) | 17 (54.8) |
| Mortality | Died | 7 (53.8) | 4 (7.8)** | 7 (20) | 4 (13.8) | 3 (25) | 8 (15.4) | 4 (8.7) | 7 (38.9)** | 7 (21.2) | 4 (12.9) |
Data are represented as frequency (percentage).
Abbreviations: LN, lymph node; ETE, Extrathyroidal extension; LVI, Lymphovascular invasion; HCV Ab, hepatitis C virus antibody.
Progression: included recurrence, relapse, and distant metastasis. A two‐sided Chi‐square test was used. Statistical significance of bold values was set at p‐value <0.05. * p < 0.05, ** p < 0.01, *** p < 0.001.
3.4. Survival analysis
As depicted in Figure 4, none of the gene or protein markers were associated with disease‐free survival. However, Kaplan–Meier curves for overall survival showed that low p53 (p < 0.001) and high PD‐L1 (p = 0.018) protein expression were associated with lower survival times. Mean survival times for each group are demonstrated in Table 4. p53 protein‐positive staining is associated with a 90% decreased risk of mortality (HR = 0.10, 95%CI = 0.02–0.47, p = 0.001), while patients with high PD‐L1 were five times more likely to die (HR = 4.74, 95%CI = 1.2–18.7, p = 0.027) (Figure 5).
FIGURE 4.
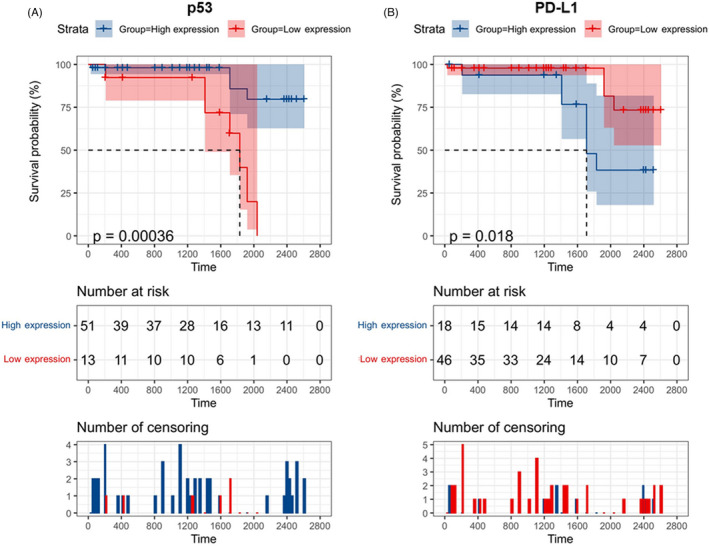
Kaplan–Meier curves for overall survival. (A) p53 protein. (B) PD‐L1 protein. Log Rank (Mantel‐Cox) test was used. Data are presented as mean survival estimates in days
TABLE 4.
Overall and disease‐free survival times of thyroid cancer patients
| Biomarker | Expression | Disease‐free survival (months) | p‐value | Overall survival (months) | p‐value |
|---|---|---|---|---|---|
| PVT1 gene | Low | 48.8 (29.5–68.2) | 0.96 | 69.6 (57.1–82.3) | 0.91 |
| High | 49.6 (38.0–61.2) | 73.8 (66.9–80.7) | |||
| p53 protein | Low | 39.1 (24.6–53.6) | 0.71 | 55.5 (45.8–65.2) | <0.001 |
| High | 51.7 (6.06–39.8) | 80.2 (74.2–86.3) | |||
| bcl2 protein | Low | 51.7 (38.4–64.9) | 0.49 | 73.5 (65.2–81.7) | 0.95 |
| High | 45.4 (30.9–59.9) | 71.1 (60.8–81.3) | |||
| PD−1 protein | Low | 55.5 (39.1–71.9) | 0.58 | 77.7 (71.7–83.6) | 0.28 |
| High | 49.3 (37.1–61.5) | 71.5 (62.9–80.1) | |||
| PD‐L1 tumor | Low | 50.9 (38.8–63.0) | 0.73 | 79.8(73.6–86.1) | 0.018 |
| High | 48.5 (30.1–66.8) | 62.9 (51.7–74.1) | |||
| PD‐L1 lymphocyte | Low | 59.7 (45.6–73.7) | 0.08 | 71.9 (62.9–80.9) | 0.63 |
| High | 39.6 (26.6–52.5) | 71.9 (64.6–79.1) | |||
| BRAF mutation | Negative | 41.9 (28.7–55.1) | 0.11 | 72.2 (65.2–79.3) | 0.92 |
| Positive | 57.9 (42.4–73.3) | 73.1 (61.4–84.7) |
Log Rank (Mantel‐Cox) test was used. Data are presented as mean survival estimates in months and 95% confidence interval. Bold values indicate significance at p‐value <0.05.
FIGURE 5.
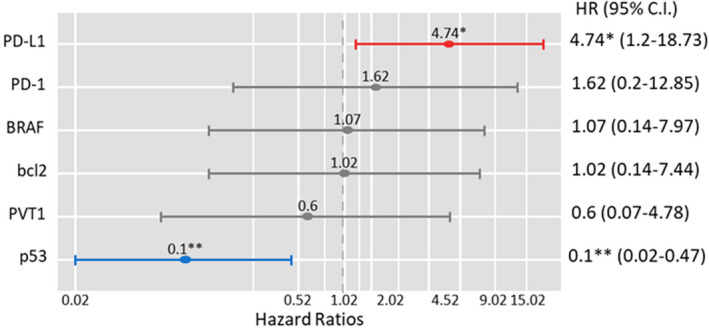
Multivariate Cox regression analysis for overall survival. Data are presented as hazard ratio (HR) and 95% confidence interval (C.I.). *p = 0.027, **p = 0.001
4. DISCUSSION
Accumulating evidence has confirmed the close relation of deregulated lncRNAs expression signature with the biological behavior of TC. 37 In this study, we selected the lncRNA PVT1 and four anti‐apoptotic proteins to determine their expression signature in TC tissues to improve our understanding of their roles in TC and their potential diagnostic and/or prognostic utility in this type of cancer. We found that PVT1 was upregulated in TC samples compared to non‐cancer tissues by overall analysis and stratified analysis by the presence of BRAF V600E mutation. This finding is congruent with the oncogenic role that PVT1 plays in several cancers, including TC. 37 , 38
The gene of PVT1 resides in a well‐known cancer risk‐related locus on "chromosome 8q24," which could partly explain the high potential of dysregulated gene expression to be associated with the development and progression of multiple cancers. 39 The oncogenic role of PVT1 has been reported in several tumors, such as lung, 40 gallbladder, 41 colon, 42 , 43 hepatocellular, 44 , 45 breast, 46 and ovarian 47 cancers.
The impact of PVT1 on cancer cell behaviors appears to be mediated via an interlinked network of other non‐coding RNAs, molecular mediators, and regulatory proteins. 48 , 49 For example, PVT1 was reported to increase Mcl‐1 (an apoptosis regulator and anti‐apoptotic factor) transcript levels by promoting its stability in renal cell carcinoma. 50 It could promote proliferation and tumorigenesis in triple‐negative breast cancer through the KLF5/β‐catenin signaling pathway. 51 PVT1 knockdown has been reported to promote apoptosis via transforming growth factor‐β signaling activation in the colorectal cancer cell. 16 In TC, PVT1 was found to modulate the proliferation of cancer cells by recruiting the "polycomb enhancer of zeste homolog 2; EZH2" and regulating the "thyroid‐stimulating hormone receptor" expression. 38 Feng et al. reported that PVT1 could act as a competing endogenous RNA (ceRNA) in papillary TC sponging the microRNA‐30a with subsequent upregulation of its target gene "insulin‐like growth factor‐1 receptor." In response to PVT1 downregulation, the later researchers found that TC cells apoptosis was promoted with inhibition of cell cycle progression, proliferation, and invasion/migration. 52
BRAF V600E, a TC‐specific gene, is one of the most common mutations that has been linked to papillary TC. 53 Although several studies have shown a subtype‐specific expression of some lncRNAs in papillary TC stratified by this mutation, such as correlation with NAMA downregulation, 54 CCND1, PERP, CDKN1A, ZMAT3, and THBS1 upregulation, 55 ENS‐653 overexpression, 56 the antisense lncRNA COMET expression, 57 and the lncRNAs AACS, ALDH3B1, ITPR3, LAD1, MMD, RASA1 and PVRL3 gene induction, 58 we did not explore a significant difference of the PVT1 expression levels between patients with TC stratified according to this type of mutation. This finding will require further confirmation by replication studies, including a larger sample size with variable TC histopathological subtypes.
On exploring the prognostic utility of the selected apoptosis‐related protein expression signatures in TC, the authors found that p53‐positive staining was associated with a 90% decreased risk of mortality. This finding aligns with the tumor suppressor functions of p53 that have long been recognized. 59 Moreover, it has been found that high expression of PVT1 in cancer cells is associated with EZH2 and MDM2 overexpression that downregulates p53 protein expression, contributing to tumorigenesis. 60 Otherwise, DNA damage and oncogenic stress could activate p53 to bind specific DNA response elements of the target genes. In TC, the tumor suppressor activity of p53 could be inhibited at the transcriptional, post‐translational protein stability, and/or downstream‐signaling level. 61 For example, downregulation of the "AT‐hook containing zinc finger protein 1 (PATZ1)" in TC can impact p53 binding to its responsive elements, favoring cell migration and epithelial‐to‐mesenchymal transition (EMT) as proposed by Chiappetta et al.. 62 Other reported molecular mechanisms as the downregulation of "Abraxas brother 1 (ABRO‐1)" that cleaves "Lys‐63‐linked ubiquitin" and upregulation of the proto‐oncogene "PTTG1‐Binding Factor (PBF) in TC enhance p53 polyubiquitination and decrease its stability. 63 , 64 Also, some "Murine double minute (MDM‐S and MDM4‐211)," truncated MDM4 spliced variants expressed in TC, were reported to be potent negative regulators to p53 in vitro. 65 All the previous reports confirm the essential role that p53 could play in the early stages of thyroid tumorigenesis and support its significant association with better survival in the current TC cases.
Our findings also indicate that overexpression of the anti‐apoptotic Bcl2 was associated with greater tumor size, and its repressed expression levels were evident in HCV‐positive cases. Bcl2 protein family has been reported to play a crucial role in the pathophysiology of TC derived from follicular epithelium by disturbing the proapoptotic/anti‐apoptotic events equilibrium. 66 Its protein expression has been identified in well‐differentiated TC subtypes compared to poorly differentiated/undifferentiated ones. 67 Using mouse models, Du et al. have explored that PVT1‐related anti‐apoptotic effect and 5‐Fu resistance in gastric cancer cases can be mediated through Bcl2 activation, confirming the potential interplay between the two studied biomolecules. 26 The observed downregulation of Bcl2 protein expression in our HCV‐positive TC cases compared to negative ones was in line with a previous study done by Visco et al. on another type of cancer. 74 The researchers compared 44 HCV‐positive cases of diffuse large B‐cell lymphoma with 132 HCV‐negative counterparts. They found that Bcl2 protein was significantly less expressed in the former cohort than the latter, suggesting that modulation of the apoptotic response via Bcl2 interacting domain degradation might be one strategy used by HCV to escape immune surveillance and ensure chronic infectivity. 68 Meanwhile, HCV nonstructural protein‐5A has been found to act as a Bcl2 homolog, interacting with Bax to protect HCC cells against apoptosis. 69
On exploring the involvement of the main "immune‐checkpoint" PD‐1/PD‐L1 in the current TC cases, we found PD‐1 protein was associated with lymph node metastasis. Furthermore, enhanced PD‐L1 expression in the tumor was associated with poor prognostic indices in terms of higher tumor stage, lymphovascular invasion, and mortality risk, as patients with high PD‐L1 protein expression were nearly five times more likely to die. Of clinical relevance, the identified predicting utility of PD‐1/PD‐L1 to worse prognosis in patients with TC, representing real hope for early implementation of anti‐PD‐1/PD‐L1 immunotherapies, contributing to "thyroid precision oncology". 70 Consistent with our findings, previous studies also reported the clinical utility of the PD‐1/PD‐L1 pathway in TC cases with/without autoimmune origin and with variable histopathological types. 71 , 72 , 73 , 74 , 75 , 76 , 77 , 78 , 79 , 80 , 81 Given the role of PD‐L1 in tumor immune escape, its expression (either at the protein or mRNA level) was associated with decreased progression‐free survival in patients with papillary TC. 71 , 73 , 81 Using "immunocompromised nude mice," PD‐L1 upregulation has been reported to confer a metastatic potential in well‐differentiated TC and its silencing delayed follicular TC growth and metastasis. 82 Interestingly, PD‐L1 was also reported to play a role in glycolysis, stemness, cell death resistance, and EMT as a major metastasis pre‐requisite. 83
5. CONCLUSION
Collectively, this study confirmed the oncogenic role of the lncRNA PVT1 in TC patients identified by overall and BRAF V600E‐stratified analyses. Furthermore, studied apoptosis‐related proteins (p53, Bcl2, and PD‐1/PD‐L1) showed associations with several poor prognostic indices in TC patients; in particular, low p53 and high PD‐L1 expressions showed significant association with low overall survival. These findings could support the prognostic utility of the studied apoptosis‐related proteins in TC and pave the road toward future personalized therapy. Multicenter studies including a larger sample size of clinical cases, particularly indeterminate lesions, are warranted to validate the findings in other histopathological TC subtypes.
CONFLICT OF INTEREST
The authors report no conflicts of interest.
AUTHOR CONTRIBUTIONS
"All authors contributed to data analysis, drafting, or revising the article, have agreed on the journal to which the article has been submitted, gave final approval of the version to be published, and agree to be accountable for all aspects of the work."
ETHICAL APPROVAL
"Declaration of Helsinki" guidelines were followed in this work. Ethical approval was provided by the Medical Research Ethics Committee, Faculty of Medicine, Suez Canal University, Egypt.
PATIENT CONSENT STATEMENT
Not applicable.
PERMISSION TO REPRODUCE MATERIAL FROM OTHER SOURCES
Not applicable.
Supporting information
Supplementary Material
ACKNOWLEDGMENT
The authors would like to acknowledge the approval and the support of this research study by grant No. (MED‐2018‐3‐9‐F‐798) from the Deanship of Scientific Research, Northern Border University, Arar, Saudi Arabia.
Ibrahiem AT, Makhdoom AK, Alanazi KS, et al. Analysis of anti‐apoptotic PVT1 oncogene and apoptosis‐related proteins (p53, Bcl2, PD‐1, and PD‐L1) expression in thyroid carcinoma. J Clin Lab Anal. 2022;36:e24390. doi: 10.1002/jcla.24390
Clinical trial registration: Not applicable.
Contributor Information
Amin K. Makhdoom, Email: akm.nbu@gmail.com.
Khalid S. Alanazi, Email: drkh.077@gmail.com.
Abdulaziz M. Alanazi, Email: Abdulaziz0Alanazi@gmail.com.
Abdulaziz M. Mukhlef, Email: Wlehamson@gmail.com.
Eman A. Toraih, Email: etoraih@tulane.edu.
Manal S. Fawzy, Email: manal.darwish@nbu.edu.sa.
DATA AVAILABILITY STATEMENT
"All data generated or analyzed during this study are included in this submitted article and supplementary materials."
REFERENCES
- 1. Rossi ED, Pantanowitz L, Hornick JL. A worldwide journey of thyroid cancer incidence centred on tumour histology. Lancet Diabetes Endocrinol. 2021;9(4):193‐194. [DOI] [PubMed] [Google Scholar]
- 2. Yang L, Tan Z, Li Y, et al. Insulin‐like growth factor 1 promotes proliferation and invasion of papillary thyroid cancer through the STAT3 pathway. J Clin Lab Anal. 2020;34(12):e23531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Zhang P, Zhang H, Dong W, et al. IL‐34 is a potential biomarker for the treatment of papillary thyroid cancer. J Clin Lab Anal. 2020;34(8):e23335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Feng K, Ma R, Li H, et al. Upregulated SLC27A2/FATP2 in differentiated thyroid carcinoma promotes tumor proliferation and migration. J Clin Lab Anal. 2021:e24148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Shan S, Wang Y, Zhu C. A comprehensive expression profile of tRNA‐derived fragments in papillary thyroid cancer. J Clin Lab Anal. 2021;35(3):e23664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Toraih EA, Elshazli RM, Trinh LN, et al. Diagnostic and prognostic performance of liquid biopsy‐derived exosomal microRNAs in thyroid cancer patients: a systematic review and meta‐analysis. Cancers. 2021;13(17):4295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Qiao DH, He XM, Deng X, et al. Aberrant expression of five miRNAs in papillary thyroid carcinomas. J Clin Lab Anal. 2021;35(9):e23907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Toraih EA, Fawzy MS, Hussein MH, et al. MicroRNA‐based risk score for predicting tumor progression following radioactive iodine ablation in well‐differentiated thyroid cancer patients: a propensity‐score matched analysis. Cancers (Basel). 2021;13(18):4649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Schmitt AM, Chang HY. Long noncoding RNAs in cancer pathways. Cancer Cell. 2016;29(4):452‐463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Gao N, Li Y, Li J, et al. Long non‐coding RNAs: the regulatory mechanisms, research strategies, and future directions in cancers. Front Oncol. 2020;10:598817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Qian Y, Shi L, Luo Z. Long non‐coding RNAs in cancer: implications for diagnosis, prognosis, and therapy. Front Med (Lausanne). 2020;7:612393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Yang JP, Yang XJ, Xiao L, Wang Y. Long noncoding RNA PVT1 as a novel serum biomarker for detection of cervical cancer. Eur Rev Med Pharmacol Sci. 2016;20(19):3980‐3986. [PubMed] [Google Scholar]
- 13. Yu J, Han J, Zhang J, et al. The long noncoding RNAs PVT1 and uc002mbe.2 in sera provide a new supplementary method for hepatocellular carcinoma diagnosis. Medicine (Baltimore). 2016;95(31):e4436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Xun C, Jiang D, Tian Z, Yunus A, Chen J. Long noncoding RNA plasmacytoma variant translocation gene 1 promotes epithelial‐mesenchymal transition in osteosarcoma. J Clin Lab Anal. 2021;35(1):e23587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Zeng C, Yu X, Lai J, Yang L, Chen S, Li Y. Overexpression of the long non‐coding RNA PVT1 is correlated with leukemic cell proliferation in acute promyelocytic leukemia. J Hematol Oncol. 2015;8:126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Takahashi Y, Sawada G, Kurashige J, et al. Amplification of PVT‐1 is involved in poor prognosis via apoptosis inhibition in colorectal cancers. Br J Cancer. 2014;110(1):164‐171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Huang C, Yu W, Wang Q, et al. Increased expression of the lncRNA PVT1 is associated with poor prognosis in pancreatic cancer patients. Minerva Med. 2015;106(3):143‐149. [PubMed] [Google Scholar]
- 18. Posa I, Carvalho S, Tavares J, Grosso AR. A pan‐cancer analysis of MYC‐PVT1 reveals CNV‐unmediated deregulation and poor prognosis in renal carcinoma. Oncotarget. 2016;7(30):47033‐47041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Liu E, Liu Z, Zhou Y. Carboplatin‐docetaxel‐induced activity against ovarian cancer is dependent on up‐regulated lncRNA PVT1. Int J Clin Exp Pathol. 2015;8(4):3803‐3810. [PMC free article] [PubMed] [Google Scholar]
- 20. Riquelme E, Suraokar MB, Rodriguez J, et al. Frequent coamplification and cooperation between C‐MYC and PVT1 oncogenes promote malignant pleural mesothelioma. J Thorac Oncol. 2014;9(7):998‐1007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Zhuang C, Li J, Liu Y, et al. Tetracycline‐inducible shRNA targeting long non‐coding RNA PVT1 inhibits cell growth and induces apoptosis in bladder cancer cells. Oncotarget. 2015;6(38):41194‐41203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Huang XM, Shi SS, Jian TM, Tang DR, Wu T, Sun FY. LncRNA PVT1 knockdown affects proliferation and apoptosis of uveal melanoma cells by inhibiting EZH2. Eur Rev Med Pharmacol Sci. 2019;23(7):2880‐2887. [DOI] [PubMed] [Google Scholar]
- 23. Wei Y, Zhang X. Transcriptome analysis of distinct long non‐coding RNA transcriptional fingerprints in lung adenocarcinoma and squamous cell carcinoma. Tumour Biol. 2016;37(12):16275‐16285. [DOI] [PubMed] [Google Scholar]
- 24. Lin T, Hou PF, Meng S, et al. Emerging roles of p53 related lncRNAs in cancer progression: a systematic review. Int J Biol Sci. 2019;15(6):1287‐1298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Olivero CE, Martínez‐Terroba E, Zimmer J, et al. p53 Activates the long noncoding RNA Pvt1b to inhibit myc and suppress tumorigenesis. Mol Cell. 2020;77(4):761‐74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Du P, Hu C, Qin Y, et al. LncRNA PVT1 mediates antiapoptosis and 5‐fluorouracil resistance via increasing Bcl2 expression in gastric cancer. J Oncol. 2019;2019:9325407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Jiang X, Wang J, Deng X, et al. Role of the tumor microenvironment in PD‐L1/PD‐1‐mediated tumor immune escape. Mol Cancer. 2019;18(1):10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Tuttle RM, Haugen B, Perrier ND. Updated American joint committee on cancer/tumor‐node‐metastasis staging system for differentiated and anaplastic thyroid cancer (Eighth Edition): what changed and why? Thyroid. 2017;27(6):751‐756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Abushouk AI, Kattan SW, Ahmedah HT, et al. Expression of oncolong noncoding RNA taurine‐upregulated gene‐1 in colon cancer: A clinical study supported by in silico analysis.. J Can Res Ther. 2021. Online ahead of print. https://www.cancerjournal.net/preprintarticle.asp?id=324167 [DOI] [PubMed] [Google Scholar]
- 30. Kattan SW, Hobani YH, Shaheen S, et al. Association of cyclin‐dependent kinase inhibitor 2B antisense RNA 1 gene expression and rs2383207 variant with breast cancer risk and survival. Cell Mol Biol Lett. 2021;26(1):14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Bustin SA, Benes V, Garson JA, et al. The MIQE guidelines: minimum information for publication of quantitative real‐time PCR experiments. Clin Chem. 2009;55(4):611‐622. [DOI] [PubMed] [Google Scholar]
- 32. Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real‐time quantitative PCR and the 2− ΔΔCT method. Methods (San Diego, Calif). 2001;25(4):402‐408. [DOI] [PubMed] [Google Scholar]
- 33. Rashid FA, Tabassum S, Khan MS, et al. VE1 immunohistochemistry is an adjunct tool for detection of BRAF. J Clin Lab Anal. 2021;35(2):e23628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Schraders M, de Jong D, Kluin P, Groenen P, van Krieken H. Lack of Bcl‐2 expression in follicular lymphoma may be caused by mutations in the BCL2 gene or by absence of the t(14;18) translocation. J Pathol. 2005;205(3):329‐335. [DOI] [PubMed] [Google Scholar]
- 35. Berntsson J, Eberhard J, Nodin B, Leandersson K, Larsson AH, Jirström K. Expression of programmed cell death protein 1 (PD‐1) and its ligand PD‐L1 in colorectal cancer: relationship with sidedness and prognosis. Oncoimmunology. 2018;7(8):e1465165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Shan T, Chen S, Wu T, Yang Y, Li S, Chen X. PD‐L1 expression in colon cancer and its relationship with clinical prognosis. Int J Clin Exp Pathol. 2019;12(5):1764‐1769. [PMC free article] [PubMed] [Google Scholar]
- 37. Peng X, Zhang K, Ma L, Xu J, Chang W. The role of long non‐coding RNAs in thyroid cancer. Front Oncol. 2020;10:941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Zhou Q, Chen J, Feng J, Wang J. Long noncoding RNA PVT1 modulates thyroid cancer cell proliferation by recruiting EZH2 and regulating thyroid‐stimulating hormone receptor (TSHR). Tumour Biol. 2016;37(3):3105‐3113. [DOI] [PubMed] [Google Scholar]
- 39. Colombo T, Farina L, Macino G, Paci P. PVT1: a rising star among oncogenic long noncoding RNAs. Biomed Res Int. 2015;2015:304208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Wang D, Hu Y. Long non‐coding RNA PVT1 competitively binds MicroRNA‐424‐5p to regulate CARM1 in radiosensitivity of non‐small‐cell lung cancer. Mol Ther Nucleic Acids. 2019;16:130‐140. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 41. Chen J, Yu Y, Li H, et al. Long non‐coding RNA PVT1 promotes tumor progression by regulating the miR‐143/HK2 axis in gallbladder cancer. Mol Cancer. 2019;18(1):33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Zhang R, Li J, Yan X, et al. Long noncoding RNA plasmacytoma variant translocation 1 (PVT1) promotes colon cancer progression via endogenous sponging miR‐26b. Med Sci Monit. 2018;24:8685‐8692. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 43. Yu X, Zhao J, He Y. Long non‐coding RNA PVT1 functions as an oncogene in human colon cancer through miR‐30d‐5p/RUNX2 axis. J BUON. 2018;23(1):48‐54. [PubMed] [Google Scholar]
- 44. Guo J, Hao C, Wang C, Li L. Long noncoding RNA PVT1 modulates hepatocellular carcinoma cell proliferation and apoptosis by recruiting EZH2. Cancer Cell Int. 2018;18:98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Xu Y, Luo X, He W, et al. Long non‐coding RNA PVT1/miR‐150/ HIG2 axis regulates the proliferation, invasion and the balance of iron metabolism of hepatocellular carcinoma. Cell Physiol Biochem. 2018;49(4):1403‐1419. [DOI] [PubMed] [Google Scholar]
- 46. Guan Y, Kuo WL, Stilwell JL, et al. Amplification of PVT1 contributes to the pathophysiology of ovarian and breast cancer. Clin Cancer Res. 2007;13(19):5745‐5755. [DOI] [PubMed] [Google Scholar]
- 47. Yang Q, Yu Y, Sun Z, Pan Y. Long non‐coding RNA PVT1 promotes cell proliferation and invasion through regulating miR‐133a in ovarian cancer. Biomed Pharmacother. 2018;106:61‐67. [DOI] [PubMed] [Google Scholar]
- 48. Wang W, Zhou R, Wu Y, et al. PVT1 promotes cancer progression via MicroRNAs. Front Oncol. 2019;9:609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Onagoruwa OT, Pal G, Ochu C, Ogunwobi OO. Oncogenic role of PVT1 and therapeutic implications. Front Oncol. 2020;10:17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Wu Q, Yang F, Yang Z, et al. Long noncoding RNA PVT1 inhibits renal cancer cell apoptosis by up‐regulating Mcl‐1. Oncotarget. 2017;8(60):101865‐101875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Tang J, Li Y, Sang Y, et al. LncRNA PVT1 regulates triple‐negative breast cancer through KLF5/beta‐catenin signaling. Oncogene. 2018;37(34):4723‐4734. [DOI] [PubMed] [Google Scholar]
- 52. Feng K, Liu Y, Xu LJ, Zhao LF, Jia CW, Xu MY. Long noncoding RNA PVT1 enhances the viability and invasion of papillary thyroid carcinoma cells by functioning as ceRNA of microRNA‐30a through mediating expression of insulin like growth factor 1 receptor. Biomed Pharmacother. 2018;104:686‐698. [DOI] [PubMed] [Google Scholar]
- 53. Enumah S, Fingeret A, Parangi S, Dias‐Santagata D, Sadow PM, Lubitz CC. BRAF. World J Surg. 2020;44(8):2685‐2691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Yoon H, He H, Nagy R, et al. Identification of a novel noncoding RNA gene, NAMA, that is downregulated in papillary thyroid carcinoma with BRAF mutation and associated with growth arrest. Int J Cancer. 2007;121(4):767‐775. [DOI] [PubMed] [Google Scholar]
- 55. Wang Q, Yang H, Wu L, et al. Identification of specific long non‐coding RNA expression: profile and analysis of association with clinicopathologic characteristics and BRAF mutation in papillary thyroid cancer. Thyroid. 2016;26(12):1719‐1732. [DOI] [PubMed] [Google Scholar]
- 56. Song B, Li R, Zuo Z, et al. LncRNA ENST00000539653 acts as an oncogenic factor via MAPK signalling in papillary thyroid cancer. BMC Cancer. 2019;19(1):297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Esposito R, Esposito D, Pallante P, Fusco A, Ciccodicola A, Costa V. Oncogenic properties of the antisense lncRNA. Cancer Res. 2019;79(9):2124‐2135. [DOI] [PubMed] [Google Scholar]
- 58. Rusinek D, Swierniak M, Chmielik E, et al. BRAFV600E‐associated gene expression profile: early changes in the transcriptome, based on a transgenic mouse model of papillary thyroid carcinoma. PLoS One. 2015;10(12):e0143688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Vousden KH, Prives C. Blinded by the light: the growing complexity of p53. Cell. 2009;137(3):413‐431. [DOI] [PubMed] [Google Scholar]
- 60. Jin K, Wang S, Zhang Y, et al. Long non‐coding RNA PVT1 interacts with MYC and its downstream molecules to synergistically promote tumorigenesis. Cell Mol Life Sci. 2019;76(21):4275‐4289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Manzella L, Stella S, Pennisi MS, et al. New insights in thyroid cancer and p53 family proteins. Int J Mol Sci. 2017;18(6):1325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Chiappetta G, Valentino T, Vitiello M, et al. PATZ1 acts as a tumor suppressor in thyroid cancer via targeting p53‐dependent genes involved in EMT and cell migration. Oncotarget. 2015;6(7):5310‐5323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Zhang J, Cao M, Dong J, et al. ABRO1 suppresses tumourigenesis and regulates the DNA damage response by stabilizing p53. Nat Commun. 2014;5:5059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Read ML, Seed RI, Fong JC, et al. The PTTG1‐binding factor (PBF/PTTG1IP) regulates p53 activity in thyroid cells. Endocrinology. 2014;155(4):1222‐1234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Prodosmo A, Giglio S, Moretti S, et al. Analysis of human MDM4 variants in papillary thyroid carcinomas reveals new potential markers of cancer properties. J Mol Med (Berl). 2008;86(5):585‐596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Gupta A, Jain S, Khurana N, Kakar AK. Expression of p63 and Bcl‐2 in malignant thyroid tumors and their correlation with other diagnostic immunocytochemical markers. J Clin Diagn Res. 2016;10(7):EC04‐EC08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Pilotti S, Collini P, Rilke F, Cattoretti G, Del Bo R, Pierotti MA. Bcl‐2 protein expression in carcinomas originating from the follicular epithelium of the thyroid gland. J Pathol. 1994;172(4):337‐342. [DOI] [PubMed] [Google Scholar]
- 68. Visco C, Wang J, Tisi MC, et al. Hepatitis C virus positive diffuse large B‐cell lymphomas have distinct molecular features and lack BCL2 translocations. Br J Cancer. 2017;117(11):1685‐1688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Chung YL, Sheu ML, Yen SH. Hepatitis C virus NS5A as a potential viral Bcl‐2 homologue interacts with Bax and inhibits apoptosis in hepatocellular carcinoma. Int J Cancer. 2003;107(1):65‐73. [DOI] [PubMed] [Google Scholar]
- 70. D'Andréa G, Lassalle S, Guevara N, Mograbi B, Hofman P. From biomarkers to therapeutic targets: the promise of PD‐L1 in thyroid autoimmunity and cancer. Theranostics. 2021;11(3):1310‐1325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Chowdhury S, Veyhl J, Jessa F, et al. Programmed death‐ligand 1 overexpression is a prognostic marker for aggressive papillary thyroid cancer and its variants. Oncotarget. 2016;7(22):32318‐32328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Fu G, Polyakova O, MacMillan C, Ralhan R, Walfish PG. Programmed death ‐ ligand 1 expression distinguishes invasive encapsulated follicular variant of papillary thyroid carcinoma from noninvasive follicular thyroid neoplasm with papillary‐like nuclear features. EBioMedicine. 2017;18:50‐55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Shi RL, Qu N, Luo TX, et al. Programmed death‐ligand 1 expression in papillary thyroid cancer and its correlation with clinicopathologic factors and recurrence. Thyroid. 2017;27(4):537‐545. [DOI] [PubMed] [Google Scholar]
- 74. Ahn S, Kim TH, Kim SW, et al. Comprehensive screening for PD‐L1 expression in thyroid cancer. Endocr Relat Cancer. 2017;24(2):97‐106. [DOI] [PubMed] [Google Scholar]
- 75. Chintakuntlawar AV, Rumilla KM, Smith CY, et al. Expression of PD‐1 and PD‐L1 in anaplastic thyroid cancer patients treated with multimodal therapy: results from a retrospective study. J Clin Endocrinol Metab. 2017;102(6):1943‐1950. [DOI] [PubMed] [Google Scholar]
- 76. Lubin D, Baraban E, Lisby A, Jalali‐Farahani S, Zhang P, Livolsi V. Papillary thyroid carcinoma emerging from hashimoto thyroiditis demonstrates increased PD‐L1 expression, which persists with metastasis. Endocr Pathol. 2018;29(4):317‐323. [DOI] [PubMed] [Google Scholar]
- 77. Gillanders SL, O'Neill JP. Prognostic markers in well differentiated papillary and follicular thyroid cancer (WDTC). Eur J Surg Oncol. 2018;44(3):286‐296. [DOI] [PubMed] [Google Scholar]
- 78. Aghajani MJ, Yang T, McCafferty CE, Graham S, Wu X, Niles N. Predictive relevance of programmed cell death protein 1 and tumor‐infiltrating lymphocyte expression in papillary thyroid cancer. Surgery. 2018;163(1):130‐136. [DOI] [PubMed] [Google Scholar]
- 79. An HJ, Ko GH, Lee JH, et al. Programmed death‐ligand 1 expression and its correlation with lymph node metastasis in papillary thyroid carcinoma. J Pathol Transl Med. 2018;52(1):9‐13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Shi X, Yu PC, Lei BW, et al. Association between programmed death‐ligand 1 expression and clinicopathological characteristics, structural recurrence, and biochemical recurrence/persistent disease in medullary thyroid carcinoma. Thyroid. 2019;29(9):1269‐1278. [DOI] [PubMed] [Google Scholar]
- 81. Ulisse S, Tuccilli C, Sorrenti S, et al. PD‐1 ligand expression in epithelial thyroid cancers: potential clinical implications. Int J Mol Sci. 2019;20(6):1405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Zhou L, Cha G, Chen L, Yang C, Xu D, Ge M. HIF1α/PD‐L1 axis mediates hypoxia‐induced cell apoptosis and tumor progression in follicular thyroid carcinoma. Onco Targets Ther. 2019;12:6461‐6470. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 83. Dong P, Xiong Y, Yue J, Hanley SJB, Watari H. Tumor‐intrinsic PD‐L1 signaling in cancer initiation, development and treatment: beyond immune evasion. Front Oncol. 2018;8:386. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Material
Data Availability Statement
"All data generated or analyzed during this study are included in this submitted article and supplementary materials."


