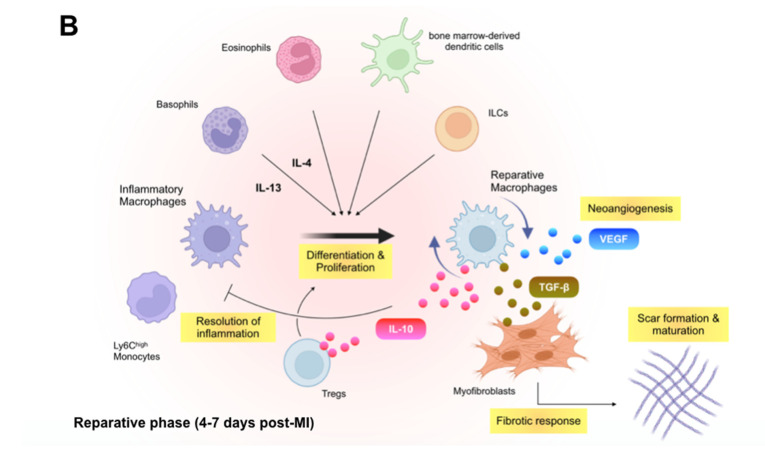
Figure 1.
Immune inflammatory response within the infarcted heart. (A): Shortly after the onset of myocardial infarction (MI), endogenous damage-associated molecular patterns (DAMPs) released from necrotic cardiomyocytes and other injured cells stimulate surviving resident cells and recruited immune cells to produce inflammatory cytokines, chemokines, and proteases (e.g., interleukin [IL]-1β, C-C motif chemokine ligand 2 [CCL2], and matrix metalloproteinases), which initiate innate immune response. Inflammatory macrophages scavenge dying cardiomyocyte-derived DNA fragments to activate interferon regulatory factor 3 (IRF3)-dependent type I interferon (IFN) signals that enhance infarct inflammation. Cardiac fibroblasts produce and release granulocyte-macrophage colony-stimulating factor (GM-CSF) in response to the engagement of their pattern recognition receptors (PRRs), which not only endows monocytes and macrophages with a pro-inflammatory signature but also stimulates bone marrow hematopoiesis. B cell-derived CCL7 further enhance monocyte accumulation in the infarcted heart. (B): In the later phase of ischemic injury, inflammatory macrophages convert their features to the anti-inflammatory phenotype. The transition is supported partly by regulatory T cells (Tregs), basophils, eosinophils, dendritic cells, and innate lymphoid cells (ILCs) and defines the reparative response in the wound healing process. Reparative macrophages as well as Tregs produce anti-inflammatory cytokines (e.g., IL-10) that resolve inflammation. The reparative macrophages likewise produce transforming growth factor (TGF)-β and vascular endothelial growth factor (VEGF), thereby promoting angiogenesis, fibrosis, and scar formation.