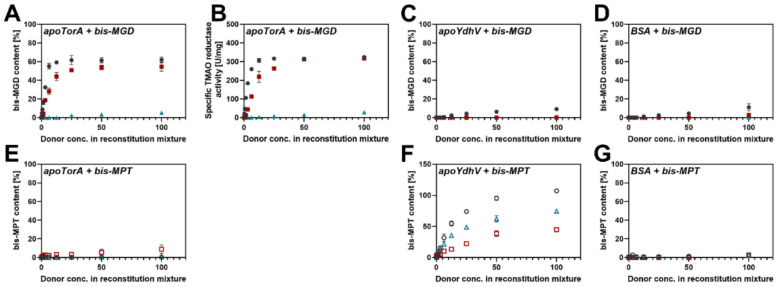
Figure 3.
Cofactor binding to apo-TorA, apo-YdhV and BSA. The bis-MGD content of 1.3 µM apo-TorA (A), apo-YdhV (C) and BSA (D) is shown after incubation with bis-MGD from different sources: bis-MGD isolated from wtTorA as donor (circles), bis-MGD formed in vitro out of bis-MPT in a MobA-catalyzed reaction (squares), residual bis-MGD after conversion into bis-MPT in a PD-catalyzed reaction (triangles). Bis-MGD was detected in form of its fluorescent oxidation product FormA-GMP. Panel (B) represents the specific TMAO reductase activity of the samples in panel A. The bis-Mo-MPT content of 1.3 µM apo-TorA (E), apo-YdhV (F) and BSA (G) is shown after incubation with bis-Mo-MPT from different sources: bis-Mo-MPT isolated from YdhV as donor (circles), residual bis-Mo-MPT after conversion into bis-MGD in a MobA-catalyzed reaction (squares), bis-Mo-MPT formed in vitro out of bis-MGD in a PD-catalyzed reaction (triangles). Bis-Mo-MPT was detected in form of its fluorescent oxidation product dephospho-FormA. No TMAO reductase activity was detected for these samples.