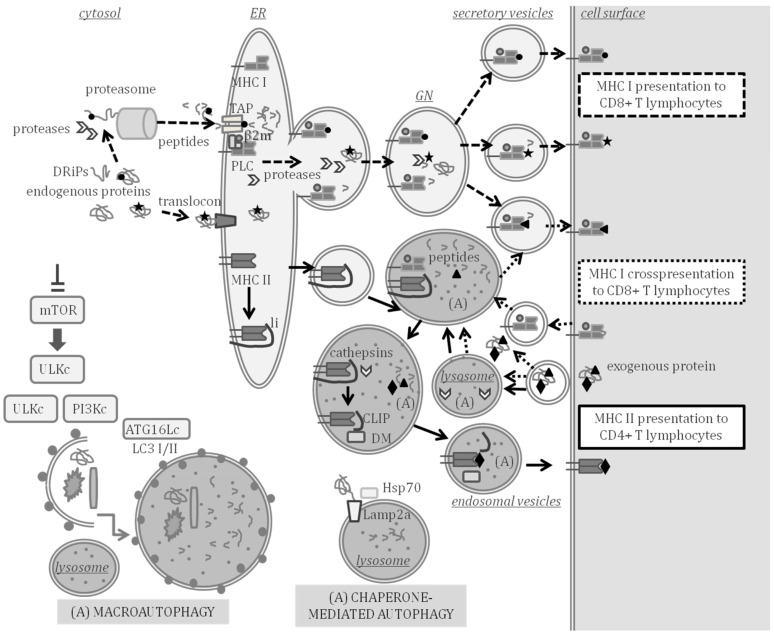
Figure 1.
Major histocompatibility complex (MHC) class I and II antigen processing and presentation (APP) and autophagy. MHC class I presents endogenous peptides produced in the cytosol (peptide represented by a circle) or in secretory compartments (star) to CD8 + T lymphocytes through the pathways indicated with the dashed arrows. Endogenously synthesized proteins and defective ribosomal products (DRiPs) are processed to peptides in the cytosol by the proteasome and cytosolic proteases. Peptides are then transported by transporter associated to antigen processing (TAP) to the ER where the peptide loading complex (PLC) is formed by a peptide, TAP, MHC class I, β2-microglobulin (β2m) and other assistant proteins. Endogenous proteins can enter the lumen of the ER through the translocon and be processed by proteases of the ER or the Golgi network (GN), thus, producing peptides that can also be loaded on MHC class I beyond the ER. Loaded MHC class I molecules are transported to the cell surface in secretory vesicles. MHC class I can also cross present peptides and prime CD8+ T lymphocytes through the pathway indicated with the dotted arrows. Cross-presented peptides (triangle) are produced by processing exogenous antigens in the cytosol or in secretory or endocytic compartments in which autophagy (A) may take place. MHC class II presents peptides (diamond) processed from exogenous proteins to CD4 + T lymphocytes through the pathway indicated with the continuous lined arrows. In the ER, MHC class II molecules are assembled and bind to the invariant chain li. The MHC class II is exported to endosomal compartments, in which autophagy can take place and where class II-associated invariant chain peptide (CLIP) remains bound to MHC class II after cleavage of li by lysosomal cathepsins. HLA-DM assists in the exchange of CLIP by a peptide that is exported to the cell surface through exocytic vesicles. Chaperone-mediated autophagy allows the targeting of cytosolic proteins to be degraded in lysosomes and is assisted by Lamp2a, Hsp70 and other chaperones. Macroautophagy is the engulfment of diverse cytosolic material to be degraded, induced by inhibition of the mammalian target of rapamycin (mTOR). Several multiprotein complexes (ULK, PI3K and ATG16L1) are involved in the formation of vesicles and lipidation of LC3-I to LC3-II, thereby, forming autophagosomes that fuse with lysosomes.