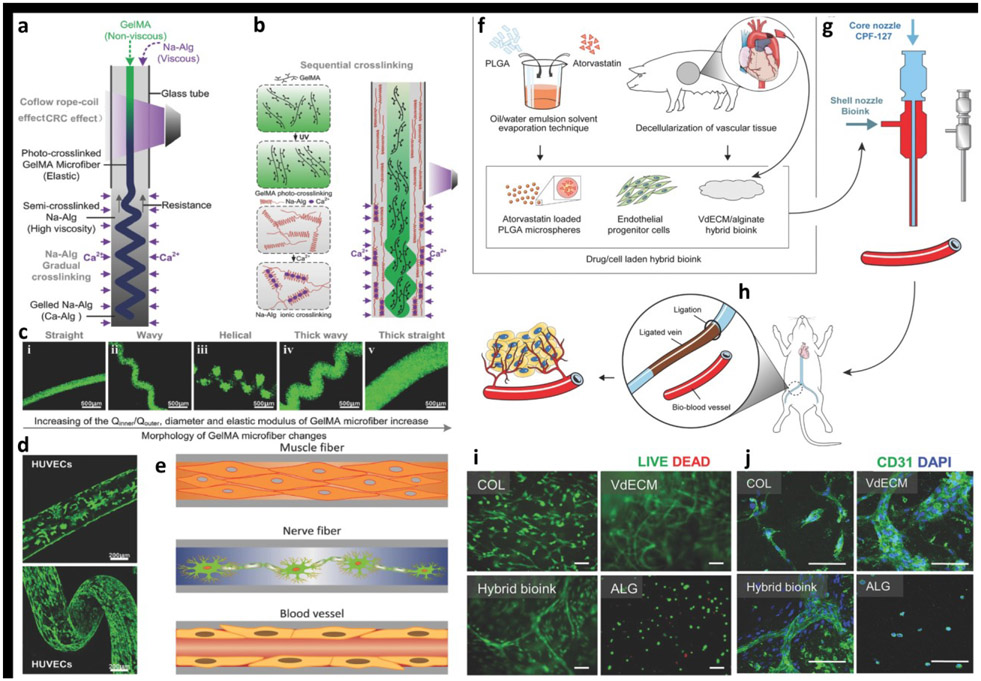
Figure 4:
a) The schematic of fabrication of the heterotypic GelMA microfibers based on the co-flow rope-coil effect. b) The sequential cross-linking strategy based on ionically crosslinked Na-Alg and photo-cross-linked GelMA. c) The morphology of GelMA microfibers changed with change in the flow rate ratio between core and sheath section of the nozzle, and the morphological change process of the GelMA microfibers was straight–wavy–helical–thick wavy–thick straight. d) Confocal laser-scanning microscopy images of the cell-laden GelMA microfibers revealed the cellular morphology after 10 days of culture. e) The potential applications of GelMA microfibers in fiber-based tissue engineering, such as muscle fibers, nerve fibers, and blood vessels. Adapted with permission from Reference [70]. f) A hybrid bioink was prepared by mixing VdECM and sodium alginate, which was used to encapsulate atorvastatin/PLGA microspheres and endothelial progenitor cells (EPCs). g) Coaxial bioprinting was applied to fabricate cell/drug-laden vascular constructs, h) which were evaluated in a mouse model by transplanting the structure to the vicinity of the ligated limb vein to treat ischemic disease. i) Relatively few dead cells were detected at Day 7 proving that the hybrid bioink provided a friendly environment to cells (scale bar: 100 μm). j) Formation of vasculature was detected in the hybrid bioink at Day 7 (Green: CD31 and Blue: 4′6-diamidino-2-phenylindole (DAPI); scale bar: 100 μm. Adapted with permission from Reference [71]).