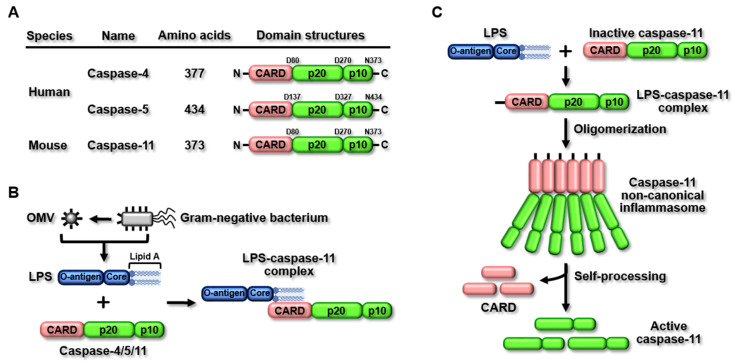
Figure 1.
Structure and activation of the caspase-11 non-canonical inflammasome. (A) Human caspase-4, caspase-5, and mouse caspase-11 have similar domain structures, consisting of an N-terminal CARD, a p20 large catalytic domain, and a C-terminal p10 small catalytic domain. (B) Sensing LPS by caspase-11. Caspase-11 recognizes LPS by direct interaction between caspase-11 CARD with LPS lipid A. (C) Activation of the caspase-11 non-canonical inflammasome. Direct interaction between LPS and caspase-11 forms LPS–caspase-11 complexes, followed by oligomerization of LPS-caspase-11 complexes by CARD–CARD interaction. CARD domains are released from the LPS–caspase-11 oligomer through self processing, resulting in the production of active caspase-11.