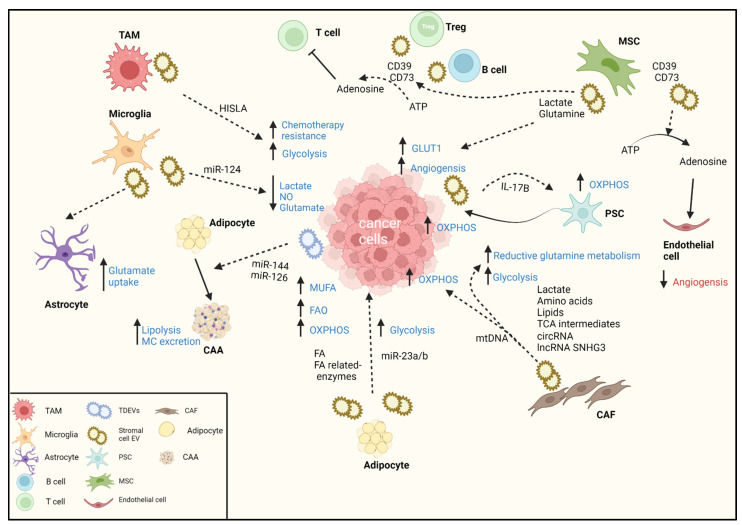
Figure 2.
Tumor microenvironment metabolic reprogramming by stromal-derived extracellular vesicles. There is crosstalk between stromal cells and cancer cells. The metabolic landscape is dynamic and depends upon the availability of nutrients and complex TME communication. This can result in opposing effects for EVs. Stromal EVs transfer different metabolites to support cancer cells. CAFs transfer amino acids and TCA cycle intermediates to cancer cells to induce glycolysis and reductive glutamine metabolism. On the other hand, CAF EVs also enhance the cancer cell OXPHOS via the transfer of mtDNA. Immune cells transfer miRNA and HISLA to augment glycolysis. MSC EVs have dual effects—supplying metabolites (lactate and glutamine) to replenish cancer cells and support angiogenesis, as well as hydrolyzing ATP to adenosine, which can inhibit angiogenesis. Adenosine hydrolyzation by B and Treg cells binds to T cells, prompting immunoevasion. Adipocytes shed EVs that can increase glycolysis but can also increase OXPHOS and FAO levels without changing that activity. TAM—tumor-associated macrophage; CAF—cancer-associated fibroblast; TME—tumor microenvironment; OXPHOS—oxidative phosphorylation; MSC—mesenchymal stem cell; PSC—pancreatic stellate cell; FA—fatty acid; FAO—fatty acid oxidation; NO—nitric oxide; TCA—tricarboxylic acid; HISLA—HIF-1α-stabilizing long noncoding RNA MC—monocarboxylic acid; CAA—cancer-associated adipocytes. Dashed arrow—transferred EV cargo.