FIGURE 4.
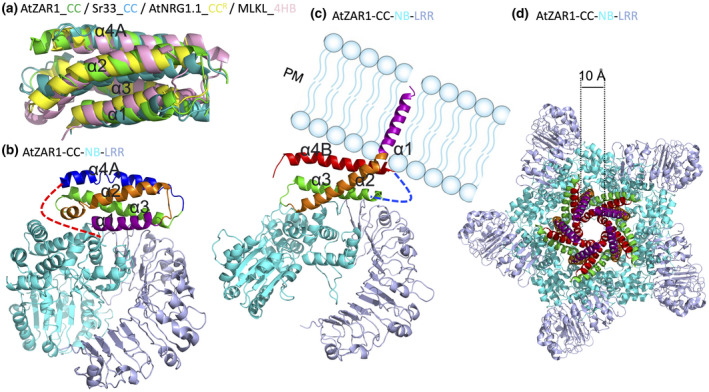
Conserved four‐helical‐bundle (4HB) fold structure of plant NLR CC and CCR domains, and the dynamics of ZAR1 resistosome formation. (a) Superimposition of AtZAR1 CC in green (PDB: 6J5W), Sr33 CC in teal (PDB: 2NCG), AtNRG1.1 CCR in yellow (PDB: 7L7W), and mouse MLKL 4HB in pink (PDB: 4BTF). (b) Structure of AtZAR1 (PDB: 6J5W) in the resting state. The four helices of the CC domain are coloured in purple, orange, green, and blue. The loop region connecting α4A and NB is shown as a curved dashed line in red, which becomes a helix termed α4B in the active state. (c) Structure of active AtZAR1 (PDB: 6J5T) shown as a monomer from the pentameric resistosome highlighting the α1 helix being flipped and penetrating the plasma membrane (PM). The α4A in the resting state becomes a potentially flexible loop region not resolved in the electron density map and is indicated as a curved dashed line in blue. The newly formed helix α4B is shown in red. (d) Structure of AtZAR1 resistosome (PDB: 6J5T) highlighting the channel pore with a diameter of about 1 nm
