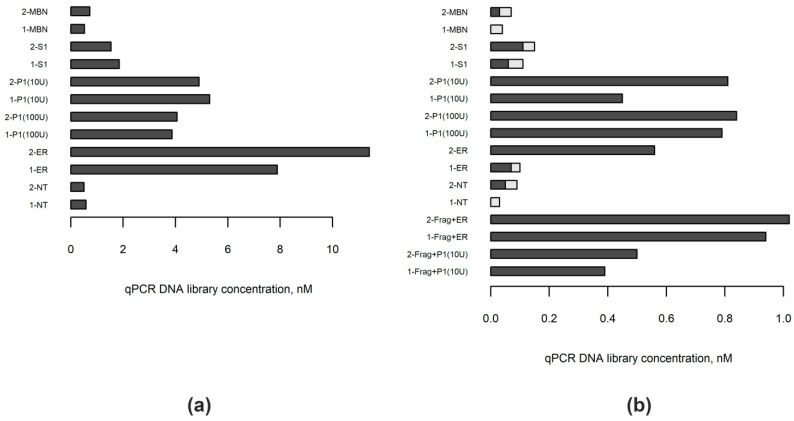
Figure 2.
Concentrations of libraries prepared from blood (n = 2) and FFPE-derived (n = 2) DNA samples with different enzymes utilized to repair DNA ends. (a) Separate aliquots of blood DNA samples 1 and 2 were enzymatically fragmented, then the ends of the resulting DNA fragments were blunted either with one of the single-strand-specific nucleases (mung bean nuclease, MBN; S1 nuclease, S1; nuclease P1, P1) or by using the standard polymerase-based approach (ER). One aliquot of each DNA sample was left untreated (NT). (b) FFPE-derived DNA samples 1 and 2 were treated similarly; however, only two aliquots of each sample were subjected to enzymatic fragmentation (Frag) before proceeding to blunt DNA ends step. The concentration of fragments with successfully ligated adaptors in each library was measured by quantitative real-time PCR (qPCR). The proportions of adapter dimers were calculated on the basis of library fragment size distribution analysis and are indicated in the picture as light grey-colored parts of the bars.