Figure 2.
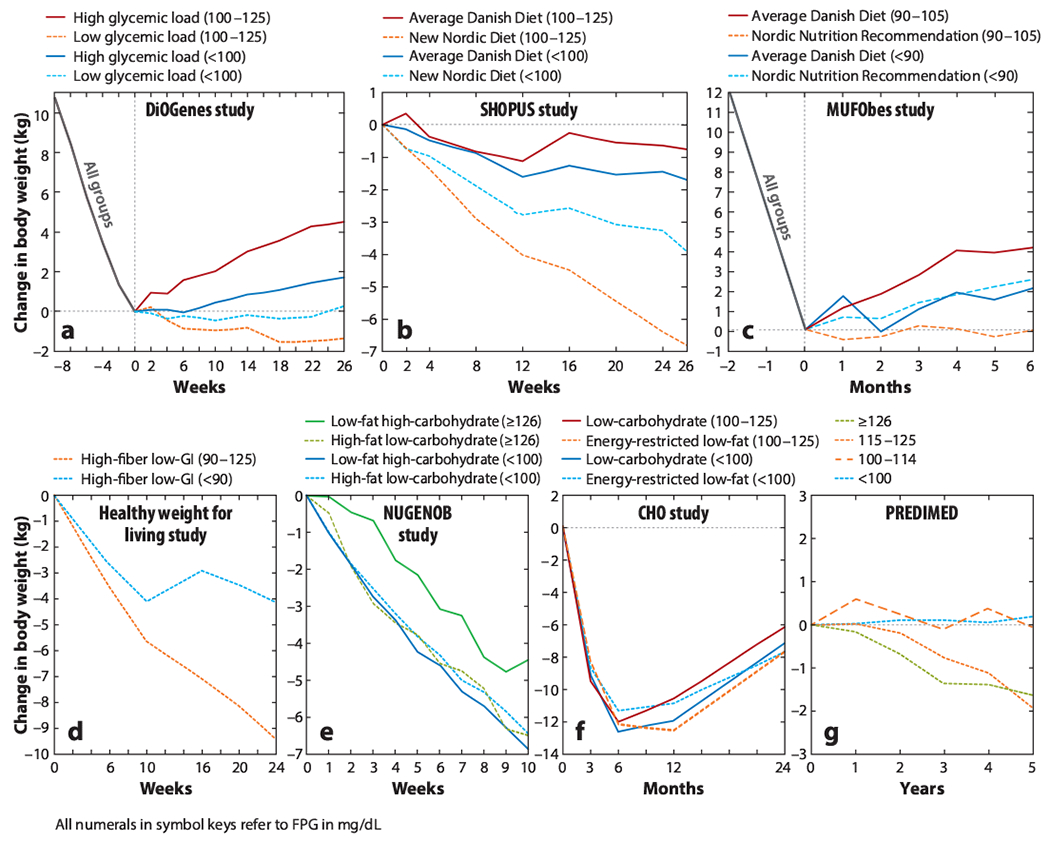
Change in body weight between groups stratified by pretreatment FPG concentrations within and between different diets. In the MUFObes study, no FPG measures exist prior to the 8-week weight-loss period. Therefore, values were used from after the 8-week weight-loss period and before randomization. The negative weeks in parts a and c indicate the weeks before intervention. The studies included are the healthy weight for living study (71), the CHO study (36), DiOGenes (34), MUFObes (33), NUGENOB (34), PREDIMED (21), and SHOPUS (34). Abbreviations: CHO, Manipulation of Carbohydrate study; DiOGenes, Diet, Obesity, and Genes trial; FPG, fasting plasma glucose; GI, glycemic index; MUFObes, Monounsaturated Fatty Acids in Obesity study; NUGENOB, Nutrient–Gene Interactions in Human Obesity trial; PREDIMED, Prevention with Mediterranean Diet study; SHOPUS, New Nordic Diet in the Supermarket Intervention trial.
