Fig. 1 |. Human liver acinus structure and organization.
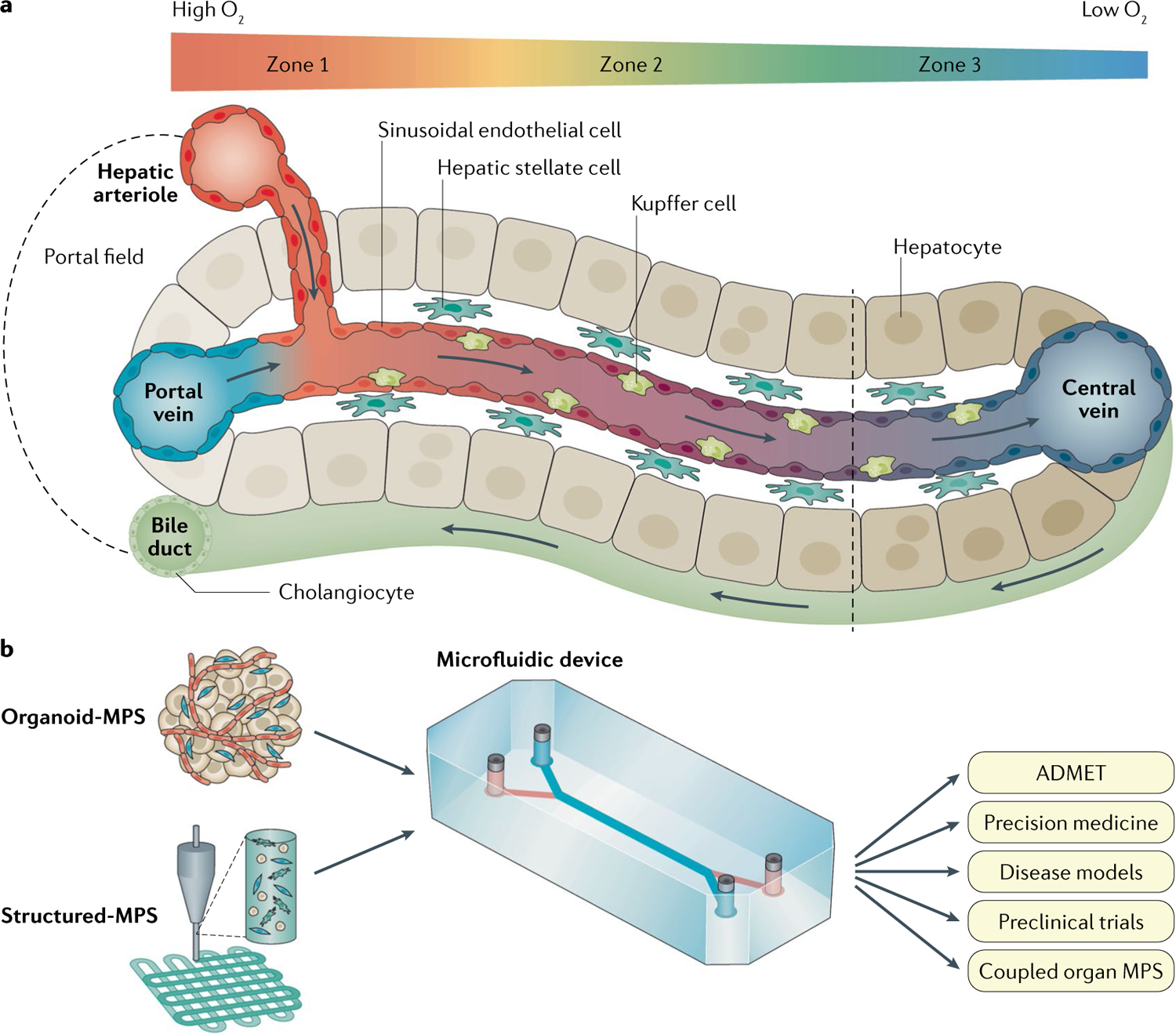
a | The liver sinusoid is created by the combined entry of blood from the nutrient-rich, oxygen-poor portal vein and the oxygen-rich, nutrient-poor hepatic arteriole. The sinusoid is lined with liver sinusoidal endothelial cells that do not form tight junctions, allowing the passage of xenobiotics, nutrients, plasma proteins, lipoproteins, gases, viruses and exosomes across the space of Disse, a thin matrix containing stellate cells, to reach cords of hepatocytes. Resident macrophages (Kupffer cells) are normally in the sinusoid. The sinusoids flow into central veins. The hepatocytes lining the space of Disse are polarized with the basal lateral surface against liver sinusoidal endothelial cells and the apical surfaces forming a network of bile canalicular spaces that link to the cholangiocytes forming the bile ducts that carry the bile produced in the hepatocytes through the biliary track and into the duodenum. A gradient of oxygen tension from high (~15–18% zone 1) to low (~5–6% zone 3) is created along the acinus due to the high rate of oxygen consumption by healthy hepatocytes. Gradients of other factors include the hormones insulin and glucagon, cytokines, and transcriptional regulators. b | The goal of one aspect of developing liver microphysiology systems (MPS) is to fully recapitulate the human liver acinus depicted in panel a. This Review discusses the evolution of the technologies and physiological cues required to make human biomimetic liver MPS based on patient-specific, stem cell-derived organoids (Organoid-MPS) and manually or bioprinted 3D structures of patient-specific stem cells and matrices (Structured-MPS). These next-generation human biomimetic liver MPS have the potential to positively affect patient-specific absorption, distribution, metabolism, excretion and toxicity (ADMET), experimental disease models for therapeutic discovery and development, precision medicine, preclinical trials, and coupled organ MPS. Although not in the scope of this Review, the knowledge gained from Organoid-MPS and Structured-MPS will also lead to the development of advanced human liver tissue derived from stem cells to create regenerative medicine treatments.
