Table 3.
Scope of Asymmetric β-Fluoride Eliminationa
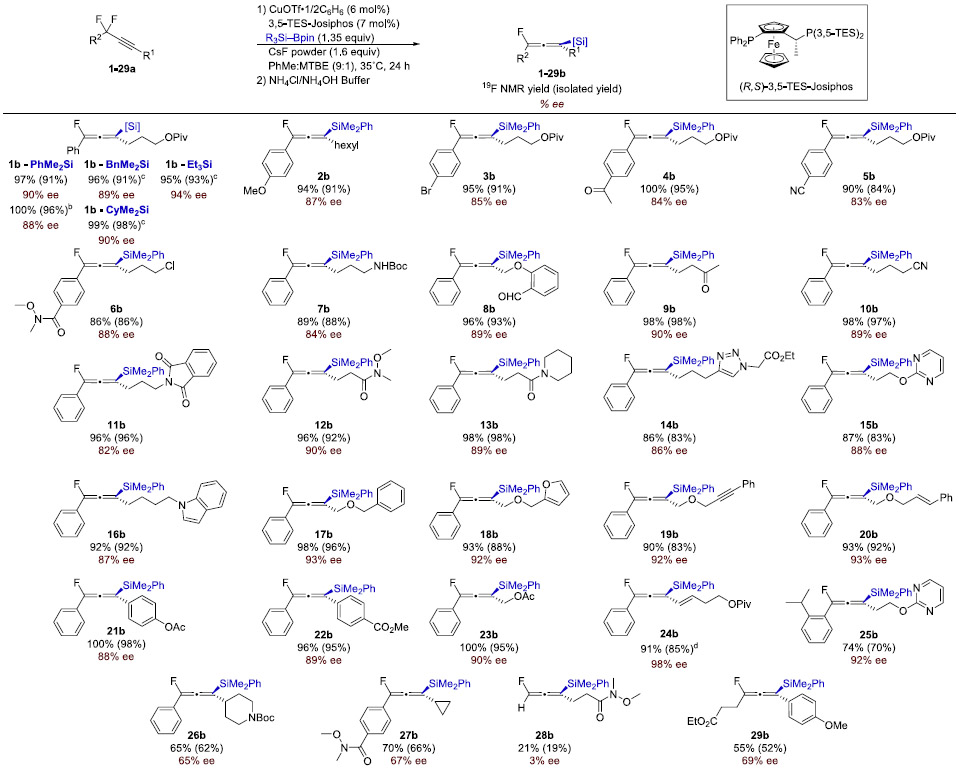
|
Standard conditions: 1–25a (0.20 mmol, 1.0 equiv), 2 (0.27 mmol, 1.35 equiv), CuOTf·½C6H6 (6 mol %), L (7 mol %), CsF (1.6–1.8 equiv), 9:1 PhMe:MTBE or MTBE (3.0 mL), 35–45 °C, 24 h. Reported yields are of isolated allene. Enantiomeric excess was determined by HPLC with a chiral stationary phase.
1a (6.0 mmol), CsF(25%)–CaF2 (1.6 equiv), 30 h.
1a (2.5–3.0 mmol), R3SiBpin (1.35–1.45 equiv), Cu (8–9 mol %), 3,5-TripJosiphos (9–10 mol %), CsF(25%)–CaF2 (1.8–2.5 equiv), MTBE, 28–45°C, 30–48 h.
NaO(2-OMeC6H4) (30 mol %), CsF (1.0 equiv), PhMe, 27 °C.
