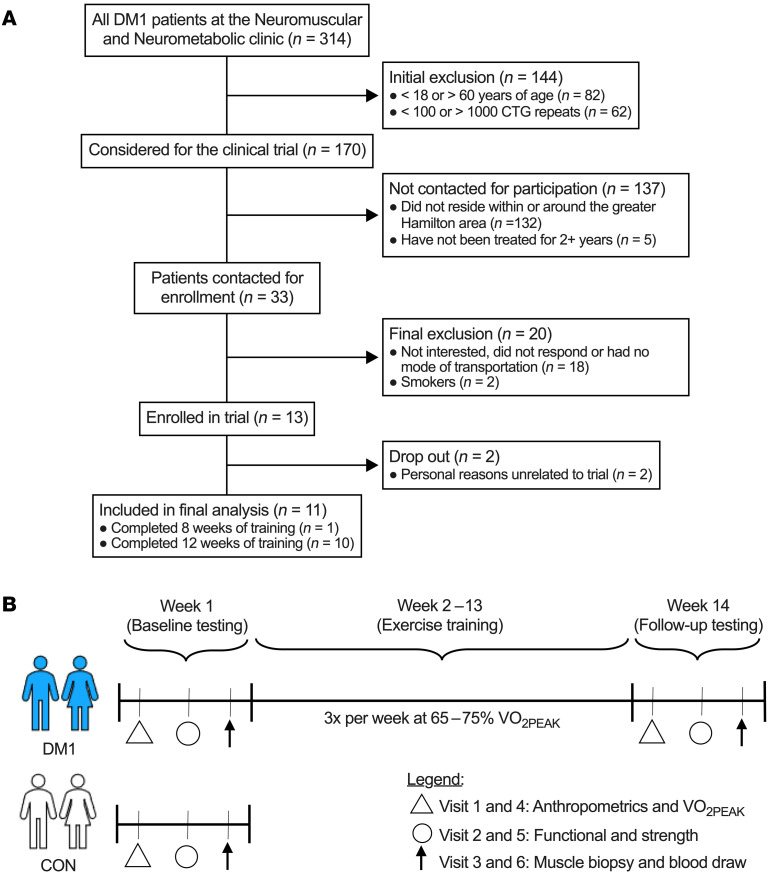
Figure 1. Flowchart of study enrollment and design.
(A) CONSORT figure of the recruitment process. All DM1 patients at the Neuromuscular and Neurometabolic Clinic at McMaster University were considered for this trial. A total of 13 patients complied with the inclusion/exclusion criteria and were interested in participating. Eleven patients were included in the final analysis. (B) Brief schematic of the study design for DM1 patients and healthy CON. DM1 patients completed the full exercise trial (visits 1–6), while CON performed baseline testing only (visits 1–3) for reference values. Visits 1 and 4 consisted of anthropometric measures, body composition assessment, electrocardiography, and cardiorespiratory fitness assessment. Visits 2 and 5 included functional testing (6-MWT, 5XSTS, and TUG tests), spirometry testing, and strength testing (maximal isometric knee extension, grip strength, and pinch grip). Finally, participants reported fasting to the laboratory for visits 3 and 6 for a blood draw and a skeletal muscle biopsy from the vastus lateralis.