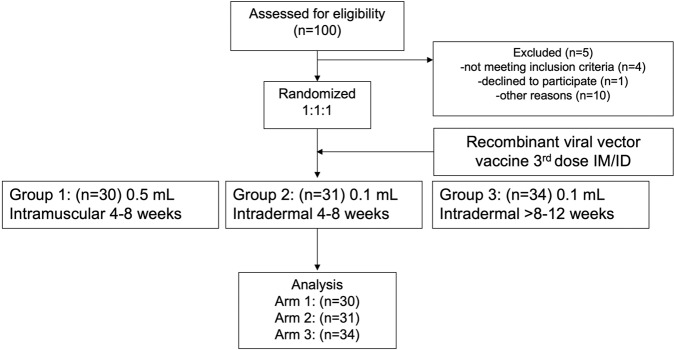
Fig. 1. CONSORT chart of study design and volunteer recruitment.
Healthy volunteers who had been vaccinated with two doses of CoronaVac were recruited into the study. 94 participants were enrolled and randomised into 3 study groups. Group 1 participants were received a full dose of ChAdOx1 nCoV-19 intramuscularly. Group 2 and Group 3 volunteers were vaccinated intradermally with a fractional dose of the viral vector vaccine. The interval between completed primary series of CoronaVac and the booster was 4–8 weeks in Group 1 and 2 but was > 8–12 weeks in Group 3. ID intradermal, IM intramuscular.