Abstract
Air pollution can adversely affect the immune response and increase the severity of the viral disease. The present study aimed to explore the relationship between symptomatology, clinical course, and inflammation markers of adult patients with coronavirus disease 2019 (COVID-19) hospitalized in Poland (n = 4432) and air pollution levels, i.e., mean 24 h and max 24 h level of benzo(a)pyrene (B(a)P) and particulate matter <10 μm (PM10) and <2.5 μm (PM2.5) during a week before their hospitalization. Exposures to PM2.5 and B(a)P exceeding the limits were associated with higher odds of early respiratory symptoms of COVID-19 and hyperinflammatory state: interleukin-6 > 100 pg/mL, procalcitonin >0.25 ng/mL, and white blood cells count >11 × 103/mL. Except for the mean 24 h PM10 level, the exceedance of other air pollution parameters was associated with increased odds for oxygen saturation <90%. Exposure to elevated PM2.5 and B(a)P levels increased the odds of oxygen therapy and death. This study evidences that worse air quality is related to increased severity of COVID-19 and worse outcome in hospitalized patients. Mitigating air pollution shall be an integral part of measures undertaken to decrease the disease burden during a pandemic of viral respiratory illness.
Keywords: Severe COVID-19, Particulate matter, benzo(a)pyrene, Inflammation markers, Epidemiology
Graphical abstract
1. Introduction
Several studies indicate the positive association between exposure to air pollutants and transmission and the severity of respiratory viral infections (Domingo and Rovira, 2020). This has been evidenced in particular for influenza, influenza-like illness, rhinovirus, and respiratory syncytial virus (RSV) infections (Nenna et al., 2017; Rodrigues et al., 2019; Wrotek et al., 2021). Air pollutants can increase susceptibility to viral infections by damaging airway epithelial cell cilia, and impair antiviral immunity by affecting different immune cell types, including macrophages, neutrophils, lymphocytes, and dendritic cells, induce oxidative stress and stimulate proinflammatory cytokine release and other inflammasome responses (Abramson et al., 2020; Barraza-Villarreal et al., 2008; Gangwar et al., 2020; Glencross et al., 2020; Yan et al., 2016; Zhao et al., 2016).
Most severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infections are asymptomatic or mild, with an infection-hospitalization rate estimated at approx. 7% (Mahajan et al., 2021). Severe coronavirus disease 2019 (COVID-19) is manifested by progressive pneumonia and acute respiratory syndrome accompanied by the release of high levels of proinflammatory cytokines, risk of thrombosis and multiple organ damage (Gu et al., 2021; Robba et al., 2020). Various risk factors have been identified, including age, obesity, male sex, and comorbidities such as cardiovascular disease, diabetes, and cancer (Booth et al., 2021; J. H.-H. Li et al., 2021; Zaher et al., 2021). Whether factors related to environmental quality can affect the severity of COVID-19 is subject to various studies. Some investigations indicate that air particulates with a diameter of ≤2.5 μm may act as SARS-CoV-2 carriers and support its spread (Borisova and Komisarenko, 2021; Khan et al., 2021; Nor et al., 2021). Moreover, the number of hospitalized and fatal patients tends to be increased in regions and periods characterized by air pollution (Bowe et al., 2021; Khan et al., 2021; Kogevinas et al., 2021; Martinez-Boubeta and Simeonidis, 2022; Paital and Agrawal, 2020). Importantly, inhalation of airborne particulate matter was shown experimentally to increase the expression of angiotensin-converting enzyme 2 (ACE2) in respiratory epithelial cells (Khan et al., 2021; Lin et al., 2018; Paital and Agrawal, 2020). As postulated, ACE2 may partially alleviate the adverse consequences of such exposure (Lin et al., 2018). However, since it also acts as the pivotal receptor for SARS-CoV-2 infection and propagation, its protective capacity may be lost during COVID-19, while exaggeration of inflammatory processes can be expected (Chaudhry et al., 2020). Therefore, it is pivotal to evaluate whether pollutants such as particulate matter <10 μm (PM10) and <2.5 μm (PM2.5) and benzo(a)pyrene (B(a)P) can be associated with changes in symptomatology, inflammation status, and clinical course and outcome of COVID-19. This is particularly relevant for regions such as Central Europe, where wood and coal combustion still plays a significant role in domestic heating and increases the emissions of PM and PM-bound polycyclic aromatic hydrocarbons such as B(a)P between late autumn and early spring (Anioł et al., 2021; Nazar and Niedoszytko, 2022). Although lockdowns associated with the COVID-19 pandemic have resulted in temporary declines in the emission of air pollutants in different world regions (Venter et al., 2020; Xu et al., 2020), the research shows that in countries such as Poland, the pollution with PM did not decrease or even significantly increased in the selected location (Rogulski and Badyda, 2021).
The present study aimed to assess the potential relationship between air pollution (PM10, PM2.5, and B(a)P levels) and the clinical course of COVID-19 in a group of Polish patients hospitalized with the severe disease between March 2020 and July 2021. To this end, the data on air quality was collected for each individual during the week preceding the hospitalization. It was hypothesized that exposure to increased levels of pollutants at the moment of infection might be associated with a different pattern of early symptoms of COVID-19 (respiratory, systemic, and anosmia), decreased oxygen saturation, and worse inflammatory profile, clinical course, and outcome.
2. Material and methods
2.1. Patients data
The clinical data for this retrospective study was retrieved from the SARSTer, a national database of hospitalized COVID-19 patients in Poland, managed by the Polish Association of Epidemiologists and Infectiologists. Overall, records of 4432 adult patients with confirmed SARS-CoV-2 infection hospitalized over the period of 17 months between March 2020 and July 2021 in one of 30 healthcare units were obtained. All of these individuals were diagnosed and treated according to the most recent Polish recommendations for the management of COVID-19 (Flisiak et al., 2020a, 2020b; 2020c; 2021a).
The demographic data included age, gender, and BMI. The data collected on early symptoms of COVID-19 were used to create three categories: (i) respiratory symptoms (cough, dyspnea), (ii) systemic symptoms (fever, headache, fatigue), (ii) anosmia. The data on baseline inflammatory markers included C-reactive protein (CRP), interleukin-6 (IL-6), procalcitonin (PCT), and white blood cell (WBC) at admission. The following values were used to define hyperinflammatory state: CRP >200 mg/L, IL-6 > 100 pg/mL, PCT >0.25 ng/mL and WBC >11 × 103/μL (Chilimuri et al., 2020; Cleland and Eranki, 2021; Flisiak et al., 2021b). The clinical data included oxygen saturation (SpO2) at admission, the need for oxygen therapy and mechanical ventilation, and outcome (survival or death).
The study had a retrospective, non-interventional nature; therefore, it did not require approval of the Ethics Committee and written consent by participants. The patients’ data were protected according to the European Union General Data Protection Regulation.
2.2. Air pollution data
Air quality data were retrieved from the Chief Inspectorate Of Environmental Protection in Poland, which is legally responsible for the national air pollution monitoring program. The following parameters were considered for this study: PM10, PM2.5, and B(a)P. Stations were equipped with instruments using the gravimetric method for PM measurement, which is considered the most accurate method (Whalley and Zandi, 2016). For each patient, each parameter's mean 24 h level during a week preceding hospitalization and the maximum 24 h level noted during this week were recorded. This period of time was selected as it takes into account the delay between infection, the onset of symptoms and hospitalization during which disruption of immune response may result in more severe disease (Faes et al., 2020, Rzymski et al., 2022). Due to the strict regionalization of COVID-19 health services in Poland, the medical units taking part in this study were admitting patients from specific residence areas for which the air quality data was collected. In the case more than one monitoring station was available in the area, the data were retrieved from all of them and averaged. The following air quality limits were considered in this study: mean 24 h and max 24 h PM10 > 50 μg/m3, mean 24 h and max 24 h PM2.5 > 20 μg/m3, and mean 24 h and max 24 h B(a)P > 1.0 ng/m3 (Directive 2004/107/EC; “Directive 2008/50/EC).
2.3. Statistical analysis
The data was analyzed with Statistica v. 13.1 (StatSoft, USA). For continuous variables, differences were tested with a Student's t-test. To evaluate associations between exceedance of air pollution limits and symptomatology, biochemical parameters, and clinical course, the classical odds ratios (ORs) with a confidence interval were calculated according to the formulas given by Bland and Altman using MedCalc (MedCalc, Ostend, Belgium). A p-value < 0.05 was considered statistically significant.
3. Results
3.1. Demographic characteristics of studied patients and air quality data
The studied group constituted adult COVID-19 patients (n = 4432), including 54.5% men, hospitalized with COVID-19 in Poland between March 2020 and June 2021. Their mean ± SD age and BMI were 61.3 ± 16.5 years and 28.3 ± 5.3 kg/m2, respectively. The majority (75.0%) suffered from at least one comorbidity. The groups exposed to air pollutants exceeding and not exceeding the limit values revealed demographic homogeneity and did not differ from each other in age, BMI, sex and presence of comorbidities. Oxygen supplementation was required by 52.4% (n = 2324) patients, while mechanical ventilation by 5.2% (n = 231). Total 478 deaths (10.8%) were recorded in the considered group.
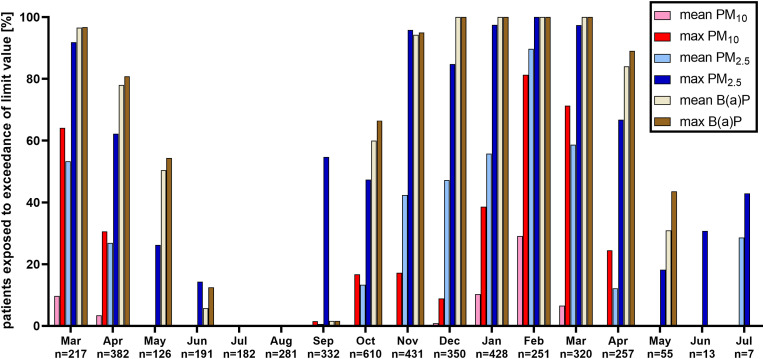
Throughout the studied period, a mean ± SD concentration of PM10, PM2.5 and B(a)P were 25.7 ± 11.0 μg/m3, 17.5 ± 9.1 μg/m3 and 2.6 ± 2.5 ng/m3, respectively. The worst air quality, indicated by the share of results exceeding the limit values, was seen between November 2020 and March 2021, with the highest percentage of exceedances of mean 24 h PM10 and PM2.5 in February 2021 (Fig. 1 ). Overall, 4.0% and 25.4% of individuals were exposed to 24 h mean and max PM10 exceeding limit levels in the week before hospitalization, respectively. This concerned 28.3% and 60.5% of patients in the case of mean and max 24 h PM2.5, respectively, and 62.5% and 64.5% in the case of 24 h mean and max B(a)P, respectively. Moreover, the exceedance of more than one limit value for daily mean and daily max air quality parameters was noted for 28.3% and 54.7% of patients, respectively.
Fig. 1.
Share of air quality levels (mean and max 24 h levels of PM10, PM2.5 and B(a)P) exceeding the limit values during a week preceding the hospitalization in the studied group of COVID-19 patients (n = 4432) hospitalized in Poland between March 2020 and July 2021.
3.2. Air pollution and early symptoms
There was an association between air pollution levels and early symptoms of COVID-19 (Table 1 ). Higher odds of respiratory manifestations were seen in patients exposed to mean 24 h PM10, mean and max 24 h PM2.5, and mean and max 24 h B(a)P exceeding the air quality limits. No effect of air pollution on the frequency of systemic symptoms onset was observed. Patients exposed to elevated max 24 h PM2.5 and mean and max 24 B(a)P revealed significantly lower odds of anosmia (Table 1).
Table 1.
The odds ratio (95% confidence interval) of a different group of symptoms in relation to exposure to air pollution parameters (mean and max 24 h levels of PM10, PM2.5 and B(a)P) exceeding limits during a week before hospitalization in the studied group of COVID-19 patients hospitalized in Poland.
| Parameter | Respiratory symptoms | p | Systemic symptoms | P | Anosmia | p |
|---|---|---|---|---|---|---|
| Mean 24 PM10 | 1.4 (0.9–2.0) | >0.05 | 0.9 (0.7–1.4) | >0.05 | 0.9 (0.6–1.5) | >0.05 |
| Max 24 h PM10 | 1.0 (0.8–1.2) | >0.05 | 0.9 (0.8–1.1) | >0.05 | 0.9 (0.7–1.1) | >0.05 |
| Mean 24 h PM2.5 | 1.3 (1.1–1.5) | <0.01 | 0.9 (0.8–1.1) | >0.05 | 0.8 (0.7–1.0) | >0.05 |
| Max 24 h PM2.5 | 1.5 (1.3–1.7) | <0.001 | 0.9 (0.8–1.1) | >0.05 | 0.7 (0.6–0.8) | <0.001 |
| Mean 24 h B(a)P | 1.5 (1.3–1.7) | <0.001 | 1.1 (0.9–1.2) | >0.05 | 0.8 (0.7–1.0) | <0.05 |
| Max 24 h B(a)P | 1.4 (1.2–1.7) | <0.001 | 1.0 (0.9–1.2) | >0.05 | 0.8 (0.6–0.9) | <0.01 |
3.3. Air pollution and inflammatory markers
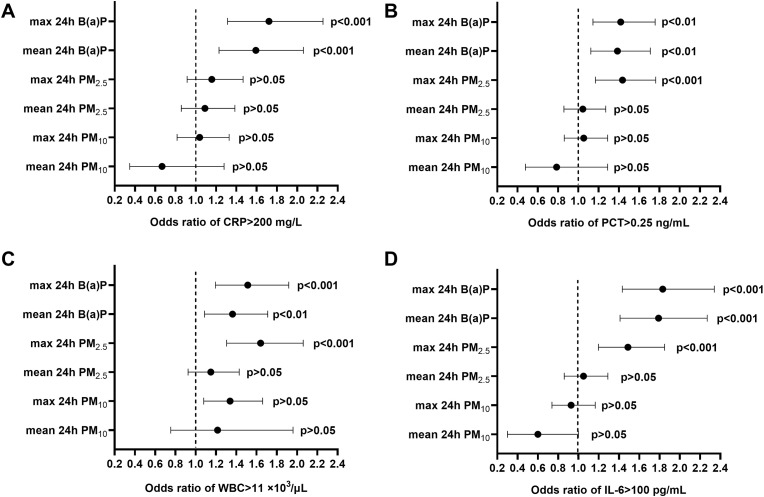
COVID-19 patients exposed to increased levels of air pollutants before hospitalization revealed distinct differences in selected inflammatory markers at admission (Table 2 ). Those exposed to max 24 h PM2.5, mean 24 h B(a)P and max 24 h B(a)P at levels exceeding the limits exhibited serum CRP concentration elevated on average by 18, 24, and 25%, respectively, while in case of serum IL-6 level, a respective increase by 44, 41 and 42% were observed. In addition, a slightly higher WBC count (by 6%) was found in patients exposed to a max 24 h PM2.5 above the limit (Table 2). The levels of inflammatory markers in groups exposed to air pollutants exceeding and not exceeding the limit values were relatively spread out as indicated by f standard deviation values (Table 2).
Table 2.
The inflammatory markers (mean ± SD) in COVID-19 patients (n = 4432) exposed to air pollutants (mean and max 24 h levels of PM10, PM2.5 and B(a)P) exceeding/not exceeding the limits during a week before hospitalization.
| Parameter | Group | CRP [mg/L] | p | PCT [ng/mL] | p | WBC [ × 103/μL] | p | IL-6 [pg/mL] | p |
|---|---|---|---|---|---|---|---|---|---|
| mean 24 h PM10 | ≤50 μg/m3 | 78.7 ± 77.6 | >0.05 | 0.5 ± 3.8 | >0.05 | 6.9 ± 5.7 | >0.05 | 74.0 ± 183.9 | >0.05 |
| >50 μg/m3 | 66.7 ± 69.3 | 0.2 ± 0.5 | 6.5 ± 3.3 | 57.6 ± 133.8 | |||||
| max 24 h PM10 | ≤50 μg/m3 | 78.0 ± 77.5 | >0.05 | 0.5 ± 3.8 | >0.05 | 6.9 ± 6.2 | >0.05 | 77.9 ± 158.9 | >0.05 |
| >50 μg/m3 | 78.9 ± 76.8 | 0.5 ± 3.6 | 6.9 ± 3.7 | 77.1 ± 237.4 | |||||
| mean 24 h PM2.5 | ≤20 μg/m3 | 75.4 ± 76.6 | >0.05 | 0.6 ± 4.0 | >0.05 | 6.9 ± 5.1 | >0.05 | 69.8 ± 156.9 | >0.05 |
| >20 μg/m3 | 78.9 ± 76.4 | 0.5 ± 3.2 | 6.8 ± 3.4 | 79.7 ± 224.6 | |||||
| max 24 h PM2.5 | ≤20 μg/m3 | 68.6 ± 75.0 | <0.001 | 0.6 ± 4.8 | >0.05 | 6.6 ± 5.3 | <0.01 | 56.8 ± 106.6 | <0.001 |
| >20 μg/m3 | 80.8 ± 77.1 | 0.5 ± 3.1 | 7.0 ± 4.3 | 81.6 ± 210.5 | |||||
| mean 24 h B(a)P | ≤1 ng/m3 | 65.7 ± 70.9 | <0.001 | 0.6 ± 4.2 | >0.05 | 6.7 ± 5.6 | >0.05 | 56.3 ± 122.9 | <0.01 |
| >1 ng/m3 | 81.6 ± 78.2 | 0.4 ± 3.6 | 6.9 ± 4.1 | 79.5 ± 200.9 | |||||
| max 24 h B(a)P | <1 ng/m3 | 65.5 ± 70.0 | <0.001 | 0.6 ± 4.2 | >0.05 | 6.7 ± 5.7 | >0.05 | 56.0 ± 124.6 | <0.01 |
| >1 ng/m3 | 81.6 ± 78.3 | 0.5 ± 3.6 | 6.9 ± 4.1 | 79.3 ± 199.5 |
As further revealed, patients exposed to mean and max 24 h B(a)P at levels exceeding the limits had increased odds of CRP >200 mg/L. In addition, patients exposed to increased levels of mean and max 24 B(a)P and max 24 h PM2.5 revealed higher odds for PCT >0.25 ng/mL, WBC >11 × 103/μL and IL-6 > 100 pg/mL at admission (Fig. 2 ).
Fig. 2.
The odds ratio (95% confidence interval) of significantly increased biochemical parameters at admission: (A) CRP >200 mg/L, (B) PCT >0.25 ng/mL, (C) WBC >11 × 103/μL and (D) IL-6 > 100 pg/mL in relation to exposure to air pollution parameters (mean and max 24 h levels of PM10, PM2.5 and B(a)P) exceeding limits during a week before hospitalization in the studied group of COVID-19 patients hospitalized in Poland.
3.4. Air pollution and the clinical course of the disease
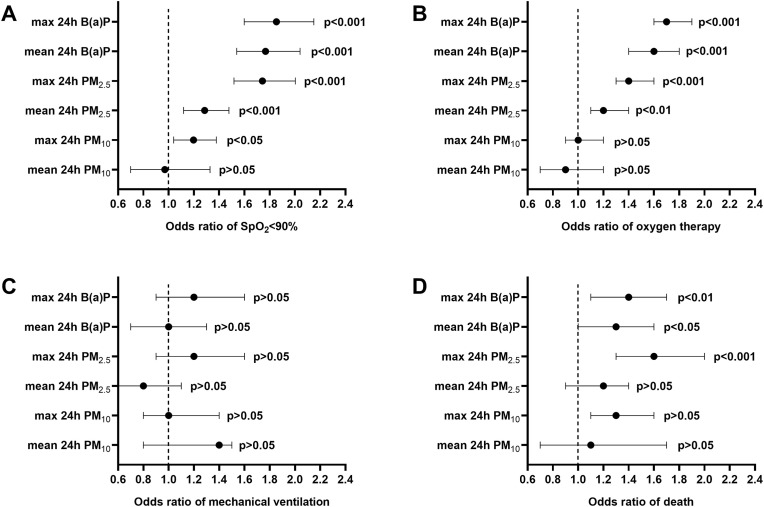
Except for the mean 24 h PM10, all other air pollution parameters had significantly increased odds for SpO2 < 90% at the admission of hospitalized patients. Exceedances of daily mean and max 24 h PM2.5 and B(a)P limits were associated with significantly increased odds for oxygen therapy. The incidence of fatal cases was higher in groups exposed to elevated max 24 h PM2.5 and mean and max 24 h B(aP) levels (Fig. 3 ). The time from hospitalization to death did not differ between patients exposed to air pollutants exceeding and not exceeding the limit levels.
Fig. 3.
The odds ratio (95% confidence interval) of (A) SpO2 < 90% at admission, (B) need for oxygen therapy, (C) mechanical ventilation, and (D) death in relation to exposure to air pollution parameters (mean and max 24 h levels of PM10, PM2.5 and B(a)P) exceeding limits during a week before hospitalization in the studied group of COVID-19 patients hospitalized in Poland.
4. Discussion
The present study, encompassing 17 months of the COVID-19 pandemic in Poland, provides evidence that air pollution is related to increased inflammation and worse prognosis in hospitalized COVID-19 patients. These findings indicate that poor environmental quality can exaggerate the COVID-19 burden. They are significant for all regions, which, similarly to Polish cities, are characterized by deteriorated air quality (European Environment Agency, 2021) and lower preparedness levels to cope with the pandemic crisis (Coccia, 2022). Therefore, reductions in emissions of air pollutants and mitigating the effects the emitted levels (e.g., by increasing residential greeness) may have on health should be an integral part of various measures undertaken to decrease the effects that an epidemic of respiratory disease can have on public health (Coccia, 2021a, 2021b; 2020a; Halabowski and Rzymski, 2020; Peng et al., 2022).
Although linkages between air pollution and increased rates of hospitalization and deaths due to COVID-19 have been already demonstrated, the previously presented results were elaborated mainly on a population level and had a correlation nature (Accarino et al., 2021; Bashir et al., 2020; Beig et al., 2020; Coker et al., 2020; Isphording and Pestel, 2021; Magazzino et al., 2020; Martinez-Boubeta and Simeonidis, 2022; Wu et al., 2020; Zhou et al., 2022). Moreover, these studies did not explore the potential associations between airborne B(a)P and COVID-19. Contrary to this, our research assessed patient-related levels of PM and B(a)P during a week before hospitalization, a time of transition of SARS-CoV-2 infection from incubation phase to symptoms onset. During this period, the antiviral defense is based on the repertoire of the innate immune response, encompassing dendritic cells, natural killer cells, macrophages, monocytes, and neutrophils, that recognize pathogen-associated and damage-associated molecular patterns to induce inflammatory signaling associated with the production of multiple interferons and cytokines (Diamond and Kanneganti, 2022; Kasuga et al., 2021). Studies indicate that dysregulation of these responses, manifested by overactivation and hyperinflammation, can significantly favor a more severe clinical course and worsen the prognosis (Blot et al., 2020; Galani and Andreakos, 2021; Janssen et al., 2021; Kapała et al., 2022; Peyneau et al., 2022). Notably, air pollutants such as PM (and PM2.5 in particular) and B(a)P are well documented to affect innate immune responses adversely. Inhalation exposure to these agents triggers inflammation in the respiratory tract and jeopardizes its function (Lewis et al., 2005; Xing et al., 2016). In the case of PM, these effects are more likely during exposure to PM2.5 than PM10 as the smaller size of this fraction allows it to penetrate the lungs more deeply, causing greater irritation of the alveolar wall (Xing et al., 2016). This may explain why in the present study, the association between COVID-19 severity was only seen for elevated levels of PM2.5. However, the potential adverse effect of PM10 fraction on the COVID-19 clinical course should not be disregarded since some research indicate that it may significantly increase the likelihood of having pneumonia (Pegoraro et al., 2021). Last but not least, exposure to PM2.5 has been shown to mediate up-regulation of receptor angiotensin-converting enzyme 2, which acts as a cellular receptor for SARS-CoV-2 as well as TMPRSS2, which is essential for proteolytic activation of this coronavirus (Borro et al., 2020; H.-H. J. Li et al., 2021). Therefore, in case of simultaneous exposure to air pollution and infection with SARS-CoV-2, the clinical severity can eventually be aggravated.
The present study clearly shows increased CRP and IL-6 in patients hospitalized a week after the mean and maximum 24 h levels of PM2.5 and B(a)P exceeded the limits. These biomarkers have prognostic value in COVID-19 (Herold et al., 2020; Liu et al., 2020). In addition, the present study has shown that exposure to elevated concentrations of PM2.5 and particularly B(a)P increase odds of hyperinflammatory state in COVID-19 manifested by CRP >200 mg, PCT >0.25 ng/mL, IL-6 > 100 μg/mL, or WBC >11 × 103/μL. This, in turn, can have a profound effect on disease outcomes if one considers that acute inflammatory response can not only promote tissue and organ failure but lead to coagulopathy and increased risk of thrombotic events (Branchford and Carpenter, 2018; Violi et al., 2021; Xiong et al., 2021). As previously shown, exposure to B(a)P can induce the production of nuclear factor kappa B and tumor necrosis factor alpha, which in turn, upregulate the IL-6 expression and promote the inflammation (Amrani et al., 2001; Malik et al., 2018; Tanabe et al., 2010; Tzeng et al., 2017). Moreover, experiments in human cells demonstrated that B(a)P metabolites (e.g., diol epoxides) inhibited the induction of interferon alpha and interferon beta (Hahon and Booth, 1986). These cytokines have antiviral and immunomodulatory activities, act as anti-inflammatory mediators, and play an important role in the initial response to viral infections (Benveniste and Qin, 2007; Biron, 1998). However, it has been shown that during SARS-CoV-2 infection, their expression is diminished (Nakhlband et al., 2021). Therefore, exposure to elevated B(a)P levels can further alter the immune response to infection and promote more severe forms of COVID-19.
In line with this, exposure to an elevated level of air pollution before hospitalization affected the odds of a worse clinical course of COVID-19 in studied patients. Exceedances of outdoor PM (including also max 24 h PM10) and B(a)P concentrations were associated with a greater frequency of SpO2 < 90% at admission, indicating the hypoxic state. Therefore, unsurprisingly, exposure to elevated PM2.5 and B(a)P increased the odds of oxygen supplementation. However, no similar association with mechanical ventilation was found. According to our epidemiological analysis, patients requiring mechanical ventilation accounted for 4.5–5% of hospitalized individuals in Poland, and this percentage was steady throughout the first 17 months of the COVID-19 pandemic (Flisiak et al., 2021c). Mechanical ventilation is necessary when previously undertaken therapeutic measures fail. The decision to use it is usually undertaken not earlier than in the second week of disease and is not only influenced by the course of COVID-19 but and by comorbidities. Moreover, in the periods of high hospital occupancy, when the availability of mechanical ventilation stations became limited, the qualification criteria had to be tightened. The lack of clear association between air pollutants and the risk of mechanical ventilation is likely due to all of these factors.
Nevertheless, odds of death were increased in patients exposed to a high level of PM2.5 and B(a)P levels. The link between air pollution and COVID-19 mortality has been explored and documented in various previous studies using population-based data and long-term air pollution monitoring results (Comunian et al., 2020; Dettori et al., 2021; Mendy et al., 2021; Semczuk-Kaczmarek et al., 2021; Wu et al., 2020). As estimated, air pollution contributed 15% to global COVID-19 mortality (Pozzer et al., 2020). Our study indicates that even short-term exposure to elevated air pollution can potentially affect the outcome of viral disease. This is in line with experimental studies in humans in which short-term exposures to an elevated level of PM induced DNA methylation changes implicated in inflammation (e.g., CD14, TLR4, TRIM45) and oxidative stress (e.g., PREX1) (Cantone et al., 2017; Li et al., 2018; Zhong et al., 2017). In rodents, even short-term exposures to elevated B(a)P levels were shown to promote lung inflammation and airway epithelial injury via Wnt5a signaling, a biomarker of pathological progression and acute respiratory distress syndrome in COVID-19 patients (Choi et al., 2020; Fan et al., 2022).
Consistently with the links between air pollution, increased inflammatory markers, and lower oxygen saturation, the present study also revealed the higher odds for early respiratory symptoms of COVID-19 in patients exposed to elevated levels of PM2.5 and B(a)P. Interestingly though, lower odds of anosmia presence were found. As previously shown, COVID-19 patients with loss of smell have a less severe clinical course and outcome of COVID-19, including these individuals who require hospitalization (Flisiak et al., 2021c; Foster et al., 2020; Talavera et al., 2020). It is suggested that the local inflammation in the area of the olfactory bulbs (which is considered to play an immunological role in preventing viral invasions) can be correlated with a more controlled antiviral response during the SARS-CoV-2 infection (Durrant et al., 2016; Gori et al., 2020; Le Bon and Horoi, 2020). In line with this, patients presenting anosmia were found to have significantly lower inflammatory markers such as CRP, PCT, and IL-6 (Coccia, 2020b, 2021c). Therefore, assuming that the exposure to air pollution is destabilizing immune responses in COVID-19, while anosmia is an indication of an appropriate immune response to viral infections in the nasopharynx, one could expect, in accordance with our observations, this symptom to be less frequent in periods of increased levels of PM and B(a)P.
Study limitations need to be highlighted. Firstly, the meteorological data, such as air temperature and humidity, was not included in this study, and as shown by other research, it may also influence the clinical course of COVID-19 (Bochenek et al., 2022; Diao et al., 2021; Fareed et al., 2021). Although the data on the wind was also not considered, changes to its speed had to be indirectly reflected in air quality levels collected from the monitoring stations since high wind speeds are known to increase air pollution, while little wind can induce stagnation of various air particulates (Coccia, 2020b, 2021c). Secondly, the study did not assess the vaccination status of the patients hospitalized during 2021. However, according to other analyses conducted in Poland, the share of vaccinated patients requiring hospitalization in the first half of 2021 was negligible (Rzymski et al., 2021). Thirdly, the study did not stratify patients according to the previously diagnosed chronic lung disease that, in some cases, could be a result or be promoted by prolonged exposure to air pollution. Last but not least, the investigated period included 17 months, during which different SARS-CoV-2 lineages were dominant in Poland. Throughout 2020 an increase in the frequency of the G superclade with hallmark D614G mutation in spike protein was observed; the beginning of 2021 was dominated by B.1.1.7 (alpha) variant, while in May 2021, the B.1.617.2 (delta) variant started to emerge (Hryhorowicz et al., 2021; Jabłońska et al., 2021). This could potentially affect selected aspects of the clinical course of severe COVID-19 (Flisiak et al., 2021c). However, the accurate data on shifts of variants could not be included in the study as the efficient nationwide genomic surveillance was not available in Poland for the substantial period considered by this study, and a very low number of SARS-CoV-2 sequences deposited by Poland institutions in the GISAID database (Furuse, 2021). On the other hand, the most evident shift in disease severity was due to the delta variant (Saito et al., 2022; Twohig et al., 2022), which was not playing a role in the majority of infections included in our study. Contrary to this, animal and epidemiological studies indicated that infection with alpha variant did not cause more severe COVID-19 compared to D614G variants (Nuñez et al., 2021). Therefore, the SARS-CoV-2 evolution appears to be insufficient in explaining the differences in the clinical parameters and outcomes found in the present study between patients exposed and not exposed to PM and B(a)P levels exceeding quality standards.
5. Conclusion
In addition to population-based studies using long-term data on air pollution monitoring, this study provides a direct link between levels of PM and B(a)P prior to hospitalization and symptomatology, inflammation, and clinical course of COVID-19. Mitigation of COVID-19 burden, and likely any future epidemics of respiratory disease, requires urgent multifaceted, short-term and long-term measures to decrease exposure to air pollution such as PM and B(a)P. This is particularly pivotal for populations inhabiting regions already highly affected by deteriorated air quality, such as Poland.
Author contributions statement
Piotr Rzymski: Conceptualization, Data curation, Formal analysis, Investigation, Methodology, Supervision, Roles/Writing – original draft, Barbara Poniedziałek: Conceptualization, Data curation, Formal analysis, Investigation, Methodology, Resources, Joanna Rosińska: Formal analysis, Investigation, Magdalena Rogalska: Investigation, Dorota Zarębska-Michaluk: Investigation, Marta Rorat: Investigation, Anna Moniuszko-Malinowska: Investigation, Beata Lorenc: Investigation, Dorota Kozielewicz: Investigation, Anna Piekarska: Investigation, Katarzyna Sikorska: Investigation, Anna Dworzańska: Investigation, Beata Bolewska: Investigation, Grzegorz Angielski: Investigation, Justyna Kowalska: Investigation, Regina Podlasin: Investigation, Barbara Oczko-Grzesik: Investigation, Włodzimierz Mazur: Investigation, Aleksandra Szymczak: Investigation, Robert Flisiak: Data curation, Investigation, Methodology, Project administration, Resources, Supervision.
Funding
This research was funded by Medical Research Agency in Poland, grant number 2020/ABM/COVID19/PTEILCHZ, Polish Association of Epidemiologists and Infectiologists and Department of Environmental Medicine (Poznan University of Medical Sciences, Poland).
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Footnotes
This paper has been recommended for acceptance by Dr. Da Chen.
References
- Abramson M.J., Wigmann C., Altug H., Schikowski T. Ambient air pollution is associated with airway inflammation in older women: a nested cross-sectional analysis. BMJ Open Respir. Res. 2020;7 doi: 10.1136/bmjresp-2019-000549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Accarino G., Lorenzetti S., Aloisio G. Assessing correlations between short-term exposure to atmospheric pollutants and COVID-19 spread in all Italian territorial areas. Environ. Pollut. 2021;268:115714. doi: 10.1016/j.envpol.2020.115714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amrani Y., Ammit A.J., Panettieri R.A., Jr. Tumor necrosis factor receptor (TNFR) 1, but not TNFR2, mediates tumor necrosis factor-alpha-induced interleukin-6 and RANTES in human airway smooth muscle cells: role of p38 and p42/44 mitogen-activated protein kinases. Mol. Pharmacol. 2001;60:646–655. [PubMed] [Google Scholar]
- Anioł E., Suder J., Bihałowicz J.S., Majewski G. The quality of air in polish health resorts with an emphasis on health on the effects of Benzo(a)pyrene in 2015–2019. Climate. 2021;9:74. [Google Scholar]
- Barraza-Villarreal A., Sunyer J., Hernandez-Cadena L., Escamilla-Nuñez M.C., Sienra-Monge J.J., Ramírez-Aguilar M., Cortez-Lugo M., Holguin F., Diaz-Sánchez D., Olin A.C., Romieu I. Air pollution, airway inflammation, and lung function in a cohort study of Mexico City schoolchildren. Environ. Health Perspect. 2008;116:832–838. doi: 10.1289/ehp.10926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bashir M.F., Ma B.J., Bilal Komal B., Bashir M.A., Farooq T.H., Iqbal N., Bashir M. Correlation between environmental pollution indicators and COVID-19 pandemic: a brief study in Californian context. Environ. Res. 2020;187:109652. doi: 10.1016/j.envres.2020.109652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beig G., Bano S., Sahu S.K., Anand V., Korhale N., Rathod A., Yadav R., Mangaraj P., Murthy B.S., Singh S., Latha R., Shinde R. COVID-19 and environmental -weather markers: unfolding baseline levels and veracity of linkages in tropical India. Environ. Res. 2020;191:110121. doi: 10.1016/j.envres.2020.110121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benveniste E.N., Qin H. Type I interferons as anti-inflammatory mediators. Sci. STKE. 2007;2007:e70. doi: 10.1126/stke.4162007pe70. [DOI] [PubMed] [Google Scholar]
- Biron C.A. Role of early cytokines, including alpha and beta interferons (IFN-alpha/beta), in innate and adaptive immune responses to viral infections. Semin. Immunol. 1998;10:383–390. doi: 10.1006/smim.1998.0138. [DOI] [PubMed] [Google Scholar]
- Blot M., Bour J.-B., Quenot J.P., Bourredjem A., Nguyen M., Guy J., Monier S., Georges M., Large A., Dargent A., Guilhem A., Mouries-Martin S., Barben J., Bouhemad B., Charles P.-E., Chavanet P., Binquet C., Piroth L., LYMPHONIE study group The dysregulated innate immune response in severe COVID-19 pneumonia that could drive poorer outcome. J. Transl. Med. 2020;18:457. doi: 10.1186/s12967-020-02646-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bochenek B., Jankowski M., Gruszczynska M., Jaczewski A., Ziemianski M., Pyrc R., Wyszogrodzki A., Nykiel G., Kopaczka D., Figurski M., Pinkas J. Weather as a potential cause of regional differences in the dynamics of COVID-19 transmission in Poland: implications for epidemic forecasting. Pol. Arch. Intern. Med. 2022;132 doi: 10.20452/pamw.16110. [DOI] [PubMed] [Google Scholar]
- Booth A., Reed A.B., Ponzo S., Yassaee A., Aral M., Plans D., Labrique A., Mohan D. Population risk factors for severe disease and mortality in COVID-19: a global systematic review and meta-analysis. PLoS One. 2021;16 doi: 10.1371/journal.pone.0247461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borisova T., Komisarenko S. Air pollution particulate matter as a potential carrier of SARS-CoV-2 to the nervous system and/or neurological symptom enhancer: arguments in favor. Environ. Sci. Pollut. Res. Int. 2021;28:40371–40377. doi: 10.1007/s11356-020-11183-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borro M., Di Girolamo P., Gentile G., De Luca O., Preissner R., Marcolongo A., Ferracuti S., Simmaco M. Evidence-based considerations exploring relations between SARS-CoV-2 pandemic and air pollution: involvement of PM2.5-mediated up-regulation of the viral receptor ACE-2. Int. J. Environ. Res. Publ. Health. 2020;17:5573. doi: 10.3390/ijerph17155573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bowe B., Xie Y., Gibson A.K., Cai M., van Donkelaar A., Martin R.V., Burnett R., Al-Aly Z. Ambient fine particulate matter air pollution and the risk of hospitalization among COVID-19 positive individuals: cohort study. Environ. Int. 2021;154:106564. doi: 10.1016/j.envint.2021.106564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Branchford B.R., Carpenter S.L. The role of inflammation in venous thromboembolism. Front. Pediatr. 2018;6:142. doi: 10.3389/fped.2018.00142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cantone L., Iodice S., Tarantini L., Albetti B., Restelli I., Vigna L., Bonzini M., Pesatori A.C., Bollati V. Particulate matter exposure is associated with inflammatory gene methylation in obese subjects. Environ. Res. 2017;152:478–484. doi: 10.1016/j.envres.2016.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaudhry F., Lavandero S., Xie X., Sabharwal B., Zheng Y.-Y., Correa A., Narula J., Levy P. Manipulation of ACE2 expression in COVID-19. Open Heart. 2020;7 doi: 10.1136/openhrt-2020-001424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chilimuri S., Sun H., Alemam A., Mantri N., Shehi E., Tejada J., Yugay A., Nayudu S.K. Predictors of mortality in adults admitted with COVID-19: retrospective cohort study from New York City. West. J. Emerg. Med. 2020;21:779–784. doi: 10.5811/westjem.2020.6.47919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi E.Y., Park H.H., Kim H., Kim H.N., Kim I., Jeon S., Kim W., Bae J.-S., Lee W. Wnt5a and Wnt11 as acute respiratory distress syndrome biomarkers for severe acute respiratory syndrome coronavirus 2 patients. Eur. Respir. J. 2020;56:2001531. doi: 10.1183/13993003.01531-2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cleland D.A., Eranki A.P. StatPearls Publishing; 2021. Procalcitonin. [PubMed] [Google Scholar]
- Coccia M. Preparedness of countries to face COVID-19 pandemic crisis: strategic positioning and factors supporting effective strategies of prevention of pandemic threats. Environ. Res. 2022;203:111678. doi: 10.1016/j.envres.2021.111678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coccia M. The impact of first and second wave of the COVID-19 pandemic in society: comparative analysis to support control measures to cope with negative effects of future infectious diseases. Environ. Res. 2021;197:111099. doi: 10.1016/j.envres.2021.111099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coccia M. High health expenditures and low exposure of population to air pollution as critical factors that can reduce fatality rate in COVID-19 pandemic crisis: a global analysis. Environ. Res. 2021;199:111339. doi: 10.1016/j.envres.2021.111339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coccia M. How do low wind speeds and high levels of air pollution support the spread of COVID-19? Atmos. Pollut. Res. 2021;12:437–445. doi: 10.1016/j.apr.2020.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coccia M. Factors determining the diffusion of COVID-19 and suggested strategy to prevent future accelerated viral infectivity similar to COVID. Sci. Total Environ. 2020;729:138474. doi: 10.1016/j.scitotenv.2020.138474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coccia M. How (Un)sustainable environments are related to the diffusion of COVID-19: the relation between Coronavirus disease 2019, air pollution, wind resource and energy. Sustainability. 2020;12:9709. [Google Scholar]
- Coker E.S., Cavalli L., Fabrizi E., Guastella G., Lippo E., Parisi M.L., Pontarollo N., Rizzati M., Varacca A., Vergalli S. The effects of air pollution on COVID-19 related mortality in Northern Italy. Environ. Resour. Econ. 2020;76:1–24. doi: 10.1007/s10640-020-00486-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Comunian S., Dongo D., Milani C., Palestini P. Air pollution and Covid-19: the role of particulate matter in the spread and increase of Covid-19's morbidity and mortality. Int. J. Environ. Res. Publ. Health. 2020;17:4487. doi: 10.3390/ijerph17124487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dettori M., Deiana G., Balletto G., Borruso G., Murgante B., Arghittu A., Azara A., Castiglia P. Air pollutants and risk of death due to COVID-19 in Italy. Environ. Res. 2021;192:110459. doi: 10.1016/j.envres.2020.110459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diamond M.S., Kanneganti T.-D. Innate immunity: the first line of defense against SARS-CoV-2. Nat. Immunol. 2022;23:165–176. doi: 10.1038/s41590-021-01091-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diao Y., Kodera S., Anzai D., Gomez-Tames J., Rashed E.A., Hirata A. Influence of population density, temperature, and absolute humidity on spread and decay durations of COVID-19: a comparative study of scenarios in China, England, Germany, and Japan. One Health. 2021;12:100203. doi: 10.1016/j.onehlt.2020.100203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Directive 2004/107/EC https://eur-lex.europa.eu/legal-content/EN/TXT/?uri=CELEX:02004L0107-20150918 [WWW Document], n.d.(accessed 2.7.22)
- Directive 2008/50/EC https://eur-lex.europa.eu/legal-content/EN/TXT/?uri=CELEX:02008L0050-20150918 [WWW Document], n.d.(accessed 2.7.22)
- Domingo J.L., Rovira J. Effects of air pollutants on the transmission and severity of respiratory viral infections. Environ. Res. 2020;187:109650. doi: 10.1016/j.envres.2020.109650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Durrant D.M., Ghosh S., Klein R.S. The olfactory bulb: an immunosensory effector organ during neurotropic viral infections. ACS Chem. Neurosci. 2016;7:464–469. doi: 10.1021/acschemneuro.6b00043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- European Environment Agency . 2021. https://www.eea.europa.eu/publications/air-quality-in-europe-2021 [Google Scholar]
- Faes C., Abrams S., Van Beckhoven D., Meyfroidt G., Vlieghe E., Hens N., Belgian Collaborative Group on COVID-19 Hospital Surveillance Time between symptom onset, hospitalisation and recovery or death: statistical analysis of Belgian COVID-19 patients. Int. J. Environ. Res. Publ. Health. 2020;17:7560. doi: 10.3390/ijerph17207560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fan L., Li W., Ma J., Cheng M., Xie L., Ye Z., Xie Y., Wang B., Yu L., Zhou Y., Chen W. Benzo(a)pyrene induces airway epithelial injury through Wnt5a-mediated non-canonical Wnt-YAP/TAZ signaling. Sci. Total Environ. 2022;815:151965. doi: 10.1016/j.scitotenv.2021.151965. [DOI] [PubMed] [Google Scholar]
- Fareed Z., Bashir M.F., Bilal Salem S. Investigating the co-movement nexus between air quality, temperature, and COVID-19 in California: implications for public health. Front. Public Health. 2021;9:815248. doi: 10.3389/fpubh.2021.815248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flisiak R., Horban A., Jaroszewicz J., Kozielewicz D., Mastalerz-Migas A., Owczuk R., Parczewski M., Pawłowska M., Piekarska A., Simon K., Tomasiewicz K., Zarębska-Michaluk D. Management of SARS-CoV-2 infection: recommendations of the polish association of Epidemiologists and Infectiologists as of April 26, 2021. Pol. Arch. Intern. Med. 2021;131:487–496. doi: 10.20452/pamw.15979. [DOI] [PubMed] [Google Scholar]
- Flisiak R., Horban A., Jaroszewicz J., Kozielewicz D., Pawłowska M., Parczewski M., Piekarska A., Simon K., Tomasiewicz K., Zarębska-Michaluk D. Management of SARS-CoV-2 infection: recommendations of the polish association of Epidemiologists and Infectiologists as of March 31, 2020. Pol. Arch. Intern. Med. 2020;130:352–357. doi: 10.20452/pamw.15270. [DOI] [PubMed] [Google Scholar]
- Flisiak R., Horban A., Jaroszewicz J., Kozielewicz D., Pawłowska M., Parczewski M., Piekarska A., Simon K., Tomasiewicz K., Zarębska-Michaluk D. Management of SARS-CoV-2 infection: recommendations of the polish association of Epidemiologists and Infectiologists. Annex no. 1 as of June 8, 2020. Pol. Arch. Intern. Med. 2020;130:557–558. doi: 10.20452/pamw.15424. [DOI] [PubMed] [Google Scholar]
- Flisiak R., Jaroszewicz J., Rogalska M., Łapiński T., Berkan-Kawińska A., Bolewska B., Tudrujek-Zdunek M., Kozielewicz D., Rorat M., Leszczyński P., Kłos K., Kowalska J., Pabjan P., Piekarska A., Mozer-Lisewska I., Tomasiewicz K., Pawłowska M., Simon K., Polanska J., Zarębska-Michaluk D. Tocilizumab improves the prognosis of COVID-19 in patients with high IL-6. J. Clin. Med. 2021;10:1583. doi: 10.3390/jcm10081583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flisiak R., Parczewski M., Horban A., Jaroszewicz J., Kozielewicz D., Pawłowska M., Piekarska A., Simon K., Tomasiewicz K., Zarębska-Michaluk D. Management of SARS-CoV-2 infection: recommendations of the polish association of Epidemiologists and Infectiologists. Annex no. 2 as of October 13, 2020. Pol. Arch. Intern. Med. 2020;130:915–918. doi: 10.20452/pamw.15658. [DOI] [PubMed] [Google Scholar]
- Flisiak R., Rzymski P., Zarębska-Michaluk D., Rogalska M., Rorat M., Czupryna P., Lorenc B., Ciechanowski P., Kozielewicz D., Piekarska A., Pokorska-Śpiewak M., Sikorska K., Tudrujek M., Bolewska B., Angielski G., Kowalska J., Podlasin R., Mazur W., Oczko-Grzesik B., Zaleska I., Szymczak A., Frańczak-Chmura P., Sobolewska-Pilarczyk M., Kłos K., Figlerowicz M., Leszczyński P., Kucharek I., Grabowski H. Demographic and clinical overview of hospitalized COVID-19 patients during the first 17 months of the pandemic in Poland. J. Clin. Med. 2021;11:117. doi: 10.3390/jcm11010117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foster K.J., Jauregui E., Tajudeen B., Bishehsari F., Mahdavinia M. Smell loss is a prognostic factor for lower severity of coronavirus disease 2019. Ann. Allergy Asthma Immunol. 2020;125:481–483. doi: 10.1016/j.anai.2020.07.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furuse Y. Genomic sequencing effort for SARS-CoV-2 by country during the pandemic. Int. J. Infect. Dis. 2021;103:305–307. doi: 10.1016/j.ijid.2020.12.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galani I.-E., Andreakos E. Impaired innate antiviral defenses in COVID-19: causes, consequences and therapeutic opportunities. Semin. Immunol. 2021;55:101522. doi: 10.1016/j.smim.2021.101522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gangwar R.S., Bevan G.H., Palanivel R., Das L., Rajagopalan S. Oxidative stress pathways of air pollution mediated toxicity: recent insights. Redox Biol. 2020;34:101545. doi: 10.1016/j.redox.2020.101545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glencross D.A., Ho T.-R., Camiña N., Hawrylowicz C.M., Pfeffer P.E. Air pollution and its effects on the immune system. Free Radic. Biol. Med. 2020;151:56–68. doi: 10.1016/j.freeradbiomed.2020.01.179. [DOI] [PubMed] [Google Scholar]
- Gori A., Leone F., Loffredo L., Cinicola B.L., Brindisi G., De Castro G., Spalice A., Duse M., Zicari A.M. COVID-19-related anosmia: the olfactory pathway hypothesis and early intervention. Front. Neurol. 2020;11:956. doi: 10.3389/fneur.2020.00956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gu S.X., Tyagi T., Jain K., Gu V.W., Lee S.H., Hwa J.M., Kwan J.M., Krause D.S., Lee A.I., Halene S., Martin K.A., Chun H.J., Hwa J. Thrombocytopathy and endotheliopathy: crucial contributors to COVID-19 thromboinflammation. Nat. Rev. Cardiol. 2021;18:194–209. doi: 10.1038/s41569-020-00469-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hahon N., Booth J.A. Benzo[a]pyrene metabolites: effects on viral interferon induction. J. Interferon Res. 1986;6:591–602. doi: 10.1089/jir.1986.6.591. [DOI] [PubMed] [Google Scholar]
- Halabowski D., Rzymski P. Taking a lesson from the COVID-19 pandemic: preventing the future outbreaks of viral zoonoses through a multi-faceted approach. Sci. Total Environ. 2020:143723. doi: 10.1016/j.scitotenv.2020.143723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herold T., Jurinovic V., Arnreich C., Lipworth B.J., Hellmuth J.C., von Bergwelt-Baildon M., Klein M., Weinberger T. Elevated levels of IL-6 and CRP predict the need for mechanical ventilation in COVID-19. J. Allergy Clin. Immunol. 2020;146:128–136.e4. doi: 10.1016/j.jaci.2020.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hryhorowicz S., Ustaszewski A., Kaczmarek-Ryś M., Lis E., Witt M., Pławski A., Ziętkiewicz E. European context of the diversity and phylogenetic position of SARS-CoV-2 sequences from Polish COVID-19 patients. J. Appl. Genet. 2021;62:327–337. doi: 10.1007/s13353-020-00603-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Isphording I.E., Pestel N. Pandemic meets pollution: poor air quality increases deaths by COVID-19. J. Environ. Econ. Manag. 2021;108:102448. doi: 10.1016/j.jeem.2021.102448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jabłońska K., Aballéa S., Auquier P., Toumi M. On the association between SARS-COV-2 variants and COVID-19 mortality during the second wave of the pandemic in Europe. J. Mark. Access Health Pol. 2021;9:2002008. doi: 10.1080/20016689.2021.2002008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janssen N.A.F., Grondman I., de Nooijer A.H., Boahen C.K., Koeken V.A.C.M., Matzaraki V., Kumar V., He X., Kox M., Koenen H.J.P.M., Smeets R.L., Joosten I., Brüggemann R.J.M., Kouijzer I.J.E., van der Hoeven H.G., Schouten J.A., Frenzel T., Reijers M.H.E., Hoefsloot W., Dofferhoff A.S.M., van Apeldoorn M.J., Blaauw M.J.T., Veerman K., Maas C., Schoneveld A.H., Hoefer I.E., Derde L.P.G., van Deuren M., van der Meer J.W.M., van Crevel R., Giamarellos-Bourboulis E.J., Joosten L.A.B., van den Heuvel M.M., Hoogerwerf J., de Mast Q., Pickkers P., Netea M.G., van de Veerdonk F.L. Dysregulated innate and adaptive immune responses discriminate disease severity in COVID-19. J. Infect. Dis. 2021;223:1322–1333. doi: 10.1093/infdis/jiab065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kapała P., Versace M., Tuchendler E., Marciniak A., Guzik P. Pulmonary embolism in patients with the coronavirus disease 2019. J. Med. Sci. 2022;91 [Google Scholar]
- Kasuga Y., Zhu B., Jang K.-J., Yoo J.-S. Innate immune sensing of coronavirus and viral evasion strategies. Exp. Mol. Med. 2021;53:723–736. doi: 10.1038/s12276-021-00602-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khan Z., Ualiyeva D., Khan Asaf, Zaman N., Sapkota S., Khan Ayub, Ali B., Ghafoor D. A correlation among the COVID-19 spread, particulate matters, and angiotensin-converting enzyme 2: a review. J. Environ. Public Health. 2021;2021:5524098. doi: 10.1155/2021/5524098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kogevinas M., Castaño-Vinyals G., Karachaliou M., Espinosa A., de Cid R., Garcia-Aymerich J., Carreras A., Cortés B., Pleguezuelos V., Jiménez A., Vidal M., O'Callaghan-Gordo C., Cirach M., Santano R., Barrios D., Puyol L., Rubio R., Izquierdo L., Nieuwenhuijsen M., Dadvand P., Aguilar R., Moncunill G., Dobaño C., Tonne C. Ambient air pollution in relation to SARS-CoV-2 infection, antibody response, and COVID-19 disease: a cohort study in Catalonia, Spain (COVICAT study) Environ. Health Perspect. 2021;129:117003. doi: 10.1289/EHP9726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le Bon S.D., Horoi M. Is anosmia the price to pay in an immune-induced scorched-earth policy against COVID-19? Med. Hypotheses. 2020;143:109881. doi: 10.1016/j.mehy.2020.109881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis T.C., Robins T.G., Dvonch J.T., Keeler G.J., Yip F.Y., Mentz G.B., Lin X., Parker E.A., Israel B.A., Gonzalez L., Hill Y. Air pollution–associated changes in lung function among asthmatic children in Detroit. Environ. Health Perspect. 2005;113:1068–1075. doi: 10.1289/ehp.7533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li H., Chen R., Cai J., Cui X., Huang N., Kan H. Short-term exposure to fine particulate air pollution and genome-wide DNA methylation: a randomized, double-blind, crossover trial. Environ. Int. 2018;120:130–136. doi: 10.1016/j.envint.2018.07.041. [DOI] [PubMed] [Google Scholar]
- Li H.-H., Liu C.-C., Hsu T.-W., Lin J.-H., Hsu J.-W., Li A.F.-Y., Yeh Y.-C., Hung S.-C., Hsu H.-S. Upregulation of ACE2 and TMPRSS2 by particulate matter and idiopathic pulmonary fibrosis: a potential role in severe COVID-19. Part. Fibre Toxicol. 2021;18:11. doi: 10.1186/s12989-021-00404-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li J., Huang D.Q., Zou B., Yang H., Hui W.Z., Rui F., Yee N.T.S., Liu C., Nerurkar S.N., Kai J.C.Y., Teng M.L.P., Li X., Zeng H., Borghi J.A., Henry L., Cheung R., Nguyen M.H. Epidemiology of COVID-19: a systematic review and meta-analysis of clinical characteristics, risk factors, and outcomes. J. Med. Virol. 2021;93:1449–1458. doi: 10.1002/jmv.26424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin C.-I., Tsai C.-H., Sun Y.-L., Hsieh W.-Y., Lin Y.-C., Chen C.-Y., Lin C.-S. Instillation of particulate matter 2.5 induced acute lung injury and attenuated the injury recovery in ACE2 knockout mice. Int. J. Biol. Sci. 2018;14:253–265. doi: 10.7150/ijbs.23489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu F., Li L., Xu M., Wu J., Luo D., Zhu Y., Li B., Song X., Zhou X. Prognostic value of interleukin-6, C-reactive protein, and procalcitonin in patients with COVID-19. J. Clin. Virol. 2020;127:104370. doi: 10.1016/j.jcv.2020.104370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magazzino C., Mele M., Schneider N. The relationship between air pollution and COVID-19-related deaths: an application to three French cities. Appl. Energy. 2020;279:115835. doi: 10.1016/j.apenergy.2020.115835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahajan S., Caraballo C., Li S.-X., Dong Y., Chen L., Huston S.K., Srinivasan R., Redlich C.A., Ko A.I., Faust J.S., Forman H.P., Krumholz H.M. SARS-CoV-2 infection hospitalization rate and infection fatality rate among the non-congregate population in Connecticut. Am. J. Med. 2021;134:812–816.e2. doi: 10.1016/j.amjmed.2021.01.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malik D.-E.-S., David R.M., Gooderham N.J. Mechanistic evidence that benzo[a]pyrene promotes an inflammatory microenvironment that drives the metastatic potential of human mammary cells. Arch. Toxicol. 2018;92:3223–3239. doi: 10.1007/s00204-018-2291-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinez-Boubeta C., Simeonidis K. Airborne magnetic nanoparticles may contribute to COVID-19 outbreak: relationships in Greece and Iran. Environ. Res. 2022;204:112054. doi: 10.1016/j.envres.2021.112054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mendy A., Wu X., Keller J.L., Fassler C.S., Apewokin S., Mersha T.B., Xie C., Pinney S.M. Air pollution and the pandemic: long-term PM2.5 exposure and disease severity in COVID-19 patients. Respirology. 2021;26:1181–1187. doi: 10.1111/resp.14140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakhlband A., Fakhari A., Azizi H. Interferon-alpha position in combating with COVID-19: a systematic review. J. Med. Virol. 2021;93:5277–5284. doi: 10.1002/jmv.27072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nazar W., Niedoszytko M. Air pollution in Poland: a 2022 narrative review with focus on respiratory diseases. Int. J. Environ. Res. Publ. Health. 2022;19:895. doi: 10.3390/ijerph19020895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nenna R., Evangelisti M., Frassanito A., Scagnolari C., Pierangeli A., Antonelli G., Nicolai A., Arima S., Moretti C., Papoff P., Villa M.P., Midulla F. Respiratory syncytial virus bronchiolitis, weather conditions and air pollution in an Italian urban area: an observational study. Environ. Res. 2017;158:188–193. doi: 10.1016/j.envres.2017.06.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nor N.S.M., Yip C.W., Ibrahim N., Jaafar M.H., Rashid Z.Z., Mustafa N., Hamid H.H.A., Chandru K., Latif M.T., Saw P.E., Lin C.Y., Alhasa K.M., Hashim J.H., Nadzir M.S.M. Particulate matter (PM2.5) as a potential SARS-CoV-2 carrier. Sci. Rep. 2021;11:2508. doi: 10.1038/s41598-021-81935-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nuñez I.A., Lien C.Z., Selvaraj P., Stauft C.B., Liu S., Starost M.F., Wang T.T. mSphere; 2021. SARS-CoV-2 B.1.1.7 Infection of Syrian Hamster Does Not Cause More Severe Disease, and Naturally Acquired Immunity Confers Protection. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paital B., Agrawal P.K. Air pollution by NO2 and PM2.5 explains COVID-19 infection severity by overexpression of angiotensin-converting enzyme 2 in respiratory cells: a review. Environ. Chem. Lett. 2020;19:1–18. doi: 10.1007/s10311-020-01091-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pegoraro V., Heiman F., Levante A., Urbinati D., Peduto I. An Italian individual-level data study investigating on the association between air pollution exposure and Covid-19 severity in primary-care setting. BMC Publ. Health. 2021;21:902. doi: 10.1186/s12889-021-10949-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peng W., Kan H., Zhou L., Wang W. Residential greenness is associated with disease severity among COVID-19 patients aged over 45 years in Wuhan, China. Ecotoxicol. Environ. Saf. 2022;232:113245. doi: 10.1016/j.ecoenv.2022.113245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peyneau M., Granger V., Wicky P.-H., Khelifi-Touhami D., Timsit J.-F., Lescure F.-X., Yazdanpanah Y., Tran-Dinh A., Montravers P., Monteiro R.C., Chollet-Martin S., Hurtado-Nedelec M., de Chaisemartin L. Innate immune deficiencies are associated with severity and poor prognosis in patients with COVID-19. Sci. Rep. 2022;12:638. doi: 10.1038/s41598-021-04705-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pozzer A., Dominici F., Haines A., Witt C., Münzel T., Lelieveld J. Regional and global contributions of air pollution to risk of death from COVID-19. Cardiovasc. Res. 2020;116:2247–2253. doi: 10.1093/cvr/cvaa288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robba C., Battaglini D., Pelosi P., Rocco P.R.M. Multiple organ dysfunction in SARS-CoV-2: MODS-CoV-2. Expet Rev. Respir. Med. 2020;14:865–868. doi: 10.1080/17476348.2020.1778470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodrigues A.F., Santos A.M., Ferreira A.M., Marino R., Barreira M.E., Cabeda J.M. Year-long Rhinovirus infection is influenced by atmospheric conditions, outdoor air virus presence, and immune system-related genetic polymorphisms. Food Environ. Virol. 2019;11:340–349. doi: 10.1007/s12560-019-09397-x. [DOI] [PubMed] [Google Scholar]
- Rogulski M., Badyda A. Air pollution observations in selected locations in Poland during the lockdown related to COVID-19. Atmosphere. 2021;12:806. [Google Scholar]
- Rzymski P., Pazgan-Simon M., Simon K., Łapiński T., Zarębska-Michaluk D., Szczepańska B., Chojnicki M., Mozer-Lisewska I., Flisiak R. Clinical characteristics of hospitalized COVID-19 patients who received at least one dose of COVID-19 vaccine. Vaccines (Basel) 2021;9:781. doi: 10.3390/vaccines9070781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rzymski Piotr, Poniedziałek Barbara, Rosińska Joanna, Ciechanowski Przemysław, Peregrym Michał, Pokorska-Śpiewak Maria, Talarek Ewa, Zaleska Izabela, Frańczak-Chmura Paulina, Pilarczyk Małgorzata, Figlerowicz Magdalena, Kucharek Izabela, Flisiak Robert. Air pollution might affect the clinical course of COVID-19 in pediatric patients. Ecotoxicol. Environ. Saf. 2022 doi: 10.1016/j.ecoenv.2022.113651. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saito A., Irie T., Suzuki R., Maemura T., Nasser H., Uriu K., Kosugi Y., Shirakawa K., Sadamasu K., Kimura I., Ito J., Wu J., Iwatsuki-Horimoto K., Ito M., Yamayoshi S., Loeber S., Tsuda M., Wang L., Ozono S., Butlertanaka E.P., Tanaka Y.L., Shimizu R., Shimizu K., Yoshimatsu K., Kawabata R., Sakaguchi T., Tokunaga K., Yoshida I., Asakura H., Nagashima M., Kazuma Y., Nomura R., Horisawa Y., Yoshimura K., Takaori-Kondo A., Imai M., Genotype to Phenotype Japan (G2P-Japan) Consortium. Tanaka S., Nakagawa S., Ikeda T., Fukuhara T., Kawaoka Y., Sato K. Enhanced fusogenicity and pathogenicity of SARS-CoV-2 Delta P681R mutation. Nature. 2022;602:300–306. doi: 10.1038/s41586-021-04266-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Semczuk-Kaczmarek K., Rys-Czaporowska A., Sierdzinski J., Kaczmarek L.D., Szymanski F.M., Platek A.E. Association between air pollution and COVID-19 mortality and morbidity. Intern. Emerg. Med. 2021 doi: 10.1007/s11739-021-02834-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Talavera B., García-Azorín D., Martínez-Pías E., Trigo J., Hernández-Pérez I., Valle-Peñacoba G., Simón-Campo P., de Lera M., Chavarría-Miranda A., López-Sanz C., Gutiérrez-Sánchez M., Martínez-Velasco E., Pedraza M., Sierra Á., Gómez-Vicente B., Guerrero Á., Arenillas J.F. Anosmia is associated with lower in-hospital mortality in COVID-19. J. Neurol. Sci. 2020;419:117163. doi: 10.1016/j.jns.2020.117163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanabe K., Matsushima-Nishiwaki R., Yamaguchi S., Iida H., Dohi S., Kozawa O. Mechanisms of tumor necrosis factor-alpha-induced interleukin-6 synthesis in glioma cells. J. Neuroinflammation. 2010;7:16. doi: 10.1186/1742-2094-7-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Twohig K.A., Nyberg T., Zaidi A., Thelwall S., Sinnathamby M.A., Aliabadi S., Seaman S.R., Harris R.J., Hope R., Lopez-Bernal J., Gallagher E., Charlett A., De Angelis D., Presanis A.M., Dabrera G. COVID-19 Genomics UK (COG-UK) consortium, 2022. Hospital admission and emergency care attendance risk for SARS-CoV-2 delta (B.1.617.2) compared with alpha (B.1.1.7) variants of concern: a cohort study. Lancet Infect. Dis. 2022;22:35–42. doi: 10.1016/S1473-3099(21)00475-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tzeng H.-P., Lan K.-C., Yang T.-H., Chung M.-N., Liu S.H. Benzo[a]pyrene activates interleukin-6 induction and suppresses nitric oxide-induced apoptosis in rat vascular smooth muscle cells. PLoS One. 2017;12 doi: 10.1371/journal.pone.0178063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Venter Z.S., Aunan K., Chowdhury S., Lelieveld J. COVID-19 lockdowns cause global air pollution declines. Proc. Natl. Acad. Sci. U. S. A. 2020;117:18984–18990. doi: 10.1073/pnas.2006853117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Violi F., Pignatelli P., Cammisotto V., Carnevale R., Nocella C. COVID-19 and thrombosis: clinical features, mechanism of disease, and therapeutic implications. Kardiol. Pol. 2021;79:1197–1205. doi: 10.33963/KP.a2021.0154. [DOI] [PubMed] [Google Scholar]
- Whalley J., Zandi S. Air Quality - Measurement and Modeling. InTech; 2016. Particulate matter sampling techniques and data modelling methods. [Google Scholar]
- Wrotek A., Badyda A., Czechowski P.O., Owczarek T., Dąbrowiecki P., Jackowska T. Air pollutants' concentrations are associated with increased number of RSV hospitalizations in Polish children. J. Clin. Med. 2021;10:3224. doi: 10.3390/jcm10153224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu X., Nethery R.C., Sabath M.B., Braun D., Dominici F. Air pollution and COVID-19 mortality in the United States: strengths and limitations of an ecological regression analysis. Sci. Adv. 2020;6 doi: 10.1126/sciadv.abd4049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xing Y.-F., Xu Y.-H., Shi M.-H., Lian Y.-X. The impact of PM2.5 on the human respiratory system. J. Thorac. Dis. 2016;8:E69–E74. doi: 10.3978/j.issn.2072-1439.2016.01.19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiong X., Chi J., Gao Q. Prevalence and risk factors of thrombotic events on patients with COVID-19: a systematic review and meta-analysis. Thromb. J. 2021;19:32. doi: 10.1186/s12959-021-00284-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu K., Cui K., Young L.-H., Hsieh Y.-K., Wang Y.-F., Zhang J., Wan S. Impact of the COVID-19 event on air quality in central China. Aerosol Air Qual. Res. 2020;20:915–929. [Google Scholar]
- Yan Z., Jin Y., An Z., Liu Y., Samet J.M., Wu W. Inflammatory cell signaling following exposures to particulate matter and ozone. Biochim. Biophys. Acta. 2016;1860:2826–2834. doi: 10.1016/j.bbagen.2016.03.030. [DOI] [PubMed] [Google Scholar]
- Zaher E.A., Keller D.M., Suntharampillai N., Ujkani E., Lesiak M. Cardiovascular risk factors of poor prognosis in COVID-19 – a review. J. Med. Sci. 2021;90:e571. [Google Scholar]
- Zhao Q., Chen H., Yang T., Rui W., Liu F., Zhang F., Zhao Y., Ding W. Direct effects of airborne PM2.5 exposure on macrophage polarizations. Biochim. Biophys. Acta. 2016;1860:2835–2843. doi: 10.1016/j.bbagen.2016.03.033. [DOI] [PubMed] [Google Scholar]
- Zhong J., Karlsson O., Wang G., Li J., Guo Y., Lin X., Zemplenyi M., Sanchez-Guerra M., Trevisi L., Urch B., Speck M., Liang L., Coull B.A., Koutrakis P., Silverman F., Gold D.R., Wu T., Baccarelli A.A. B vitamins attenuate the epigenetic effects of ambient fine particles in a pilot human intervention trial. Proc. Natl. Acad. Sci. U. S. A. 2017;114:3503–3508. doi: 10.1073/pnas.1618545114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou N., Dai H., Zha W., Lv Y. The impact of meteorological factors and PM2.5 on COVID-19 transmission. Epidemiol. Infect. 2022;150:e38. doi: 10.1017/S0950268821002570. [DOI] [PMC free article] [PubMed] [Google Scholar]