Figure 1. Temporal Virus Neutralizing Antibody Responses Against Ancestral SARS-CoV-2 Strain (D614G), Delta Variant (B.1.617.2), and Omicron Variant (B.1.1.529, BA.1).
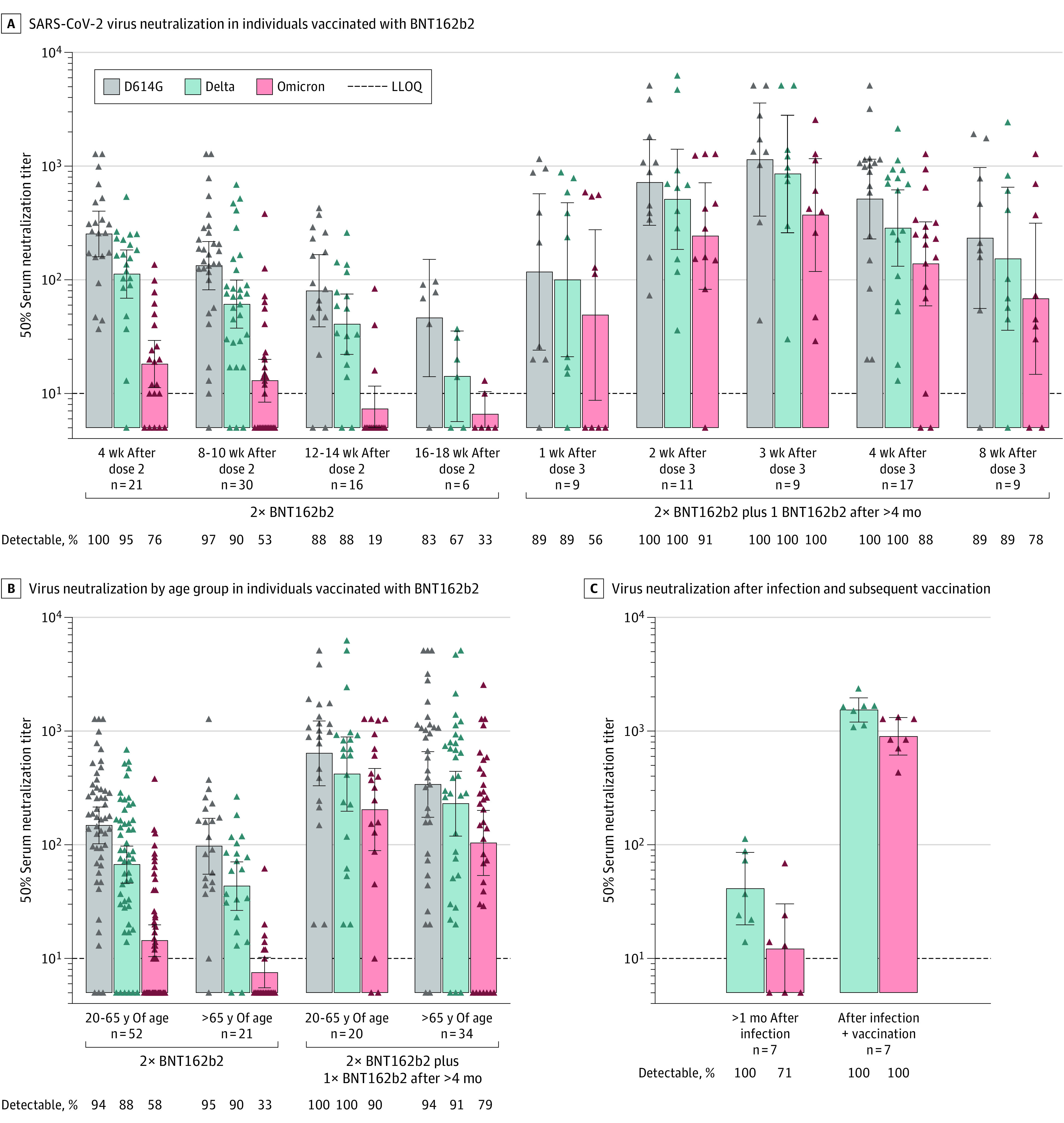
A, Live virus neutralization titers for a cross-sectional cohort of individuals vaccinated with BNT162b2 (Pfizer/BioNTech) vaccine (n = 128) at 4 to 18 weeks following the second dose in the primary 2-dose vaccination series and 1 to 8 weeks following a third BNT162b2 dose administered more than 4 months after the second dose. B, Live virus neutralization titers stratified by age group 4 to 18 weeks after the primary 2-dose BNT162b2 vaccination series and 1 to 8 weeks after the third BNT162b2 dose. C, In a longitudinal cohort of individuals (n = 7) who became infected before January 2021—before the Alpha and Delta variants became dominant in Denmark—virus neutralization titers were determined 46 to 186 days after a polymerase chain reaction positive test (median: 65 days) and after subsequent vaccination more than 6 months after the infection, primarily within 5 weeks postvaccination. The viral targets in the microneutralization assays were Danish clinical isolates passaged twice in Vero E6 cells and sequenced to confirm lineage-specific spike variations. Data points represent individual 50% serum neutralization titers. Bars represent the geometric mean titer (indicated above bar) and error bars the 95% CI. The lower limit of quantitation (LLOQ) was 10 and all values below the LLOQ were set to 0.5 times the LLOQ.
