Figure 2. Proposed models for the differentiation of cytokine-skewed T follicular helper (Tfh) cell subsets in mice and humans.
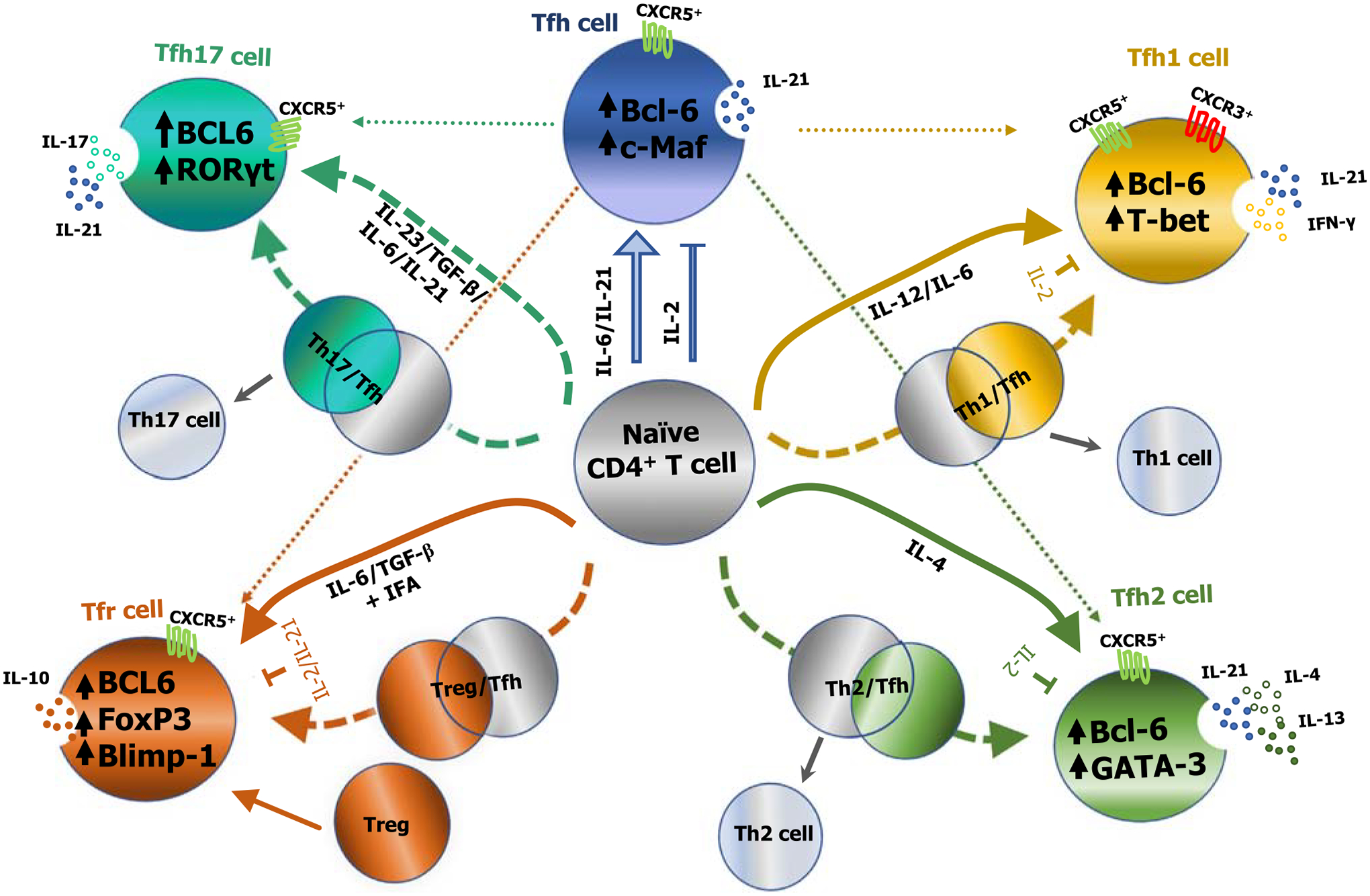
A schematic representation of the differentiation of cytokine-skewed Tfh cells from naïve CD4+ T cells is shown. External stimuli including the cytokine milieu, as well as intrinsic factors such as lineage-specific transcription factors (TFs), induce the differentiation of naïve CD4+ T cells into subsets of cytokine-skewed Tfh cell subsets. Polarizing cytokines can induce Tfh cells to coexpress transcriptional programming that is typical of other CD4+ helper T cells, in addition to Bcl-6 that is typically expressed by Tfh cells. It is possible that a partial ‘hybrid’ [T helper (Th)1/Tfh, Th2/Tfh, Th17/Tfh, and T regulatory cell (Treg)/Tfh] state occurs before maturing into different cytokine-skewed germinal center (GC)-Tfh cells. In view of this, Tfh cells can produce limited quantities of canonical cytokines that have been used to identify other T helper subsets, in addition to interleukin (IL)-21, typically associated with classical Tfh cells. The majority of T follicular regulatory (Tfr) cells arise via differentiation from infiltrating Tregs, although some also differentiate from naïve CD4+ T cells. Broken lines indicate hypothesized differentiation pathways for cytokine-skewed Tfh subsets [12]. Abbreviations: Bcl-6, B cell lymphoma-6; Blimp-1, B lymphocyte-induced maturation protein-1; c-Maf, c-musculoaponeurotic fibrosarcoma oncogene homolog; CXCR5, C-X-C chemokine receptor 5; FoxP3, forkhead box protein 3; GATA-3, GATA-binding protein 3; IFN-γ, interferon γ; RORγt, RAR-related orphan receptor γ; T-bet, T-box expressed in T cells; Tfh1, Th1-like Tfh cells; Tfh2, Th2-like Tfh cells; Tfh17, Th17-like Tfh cells.
