Figure 1 (facing page). Recruitment, Randomization, and Follow-up.
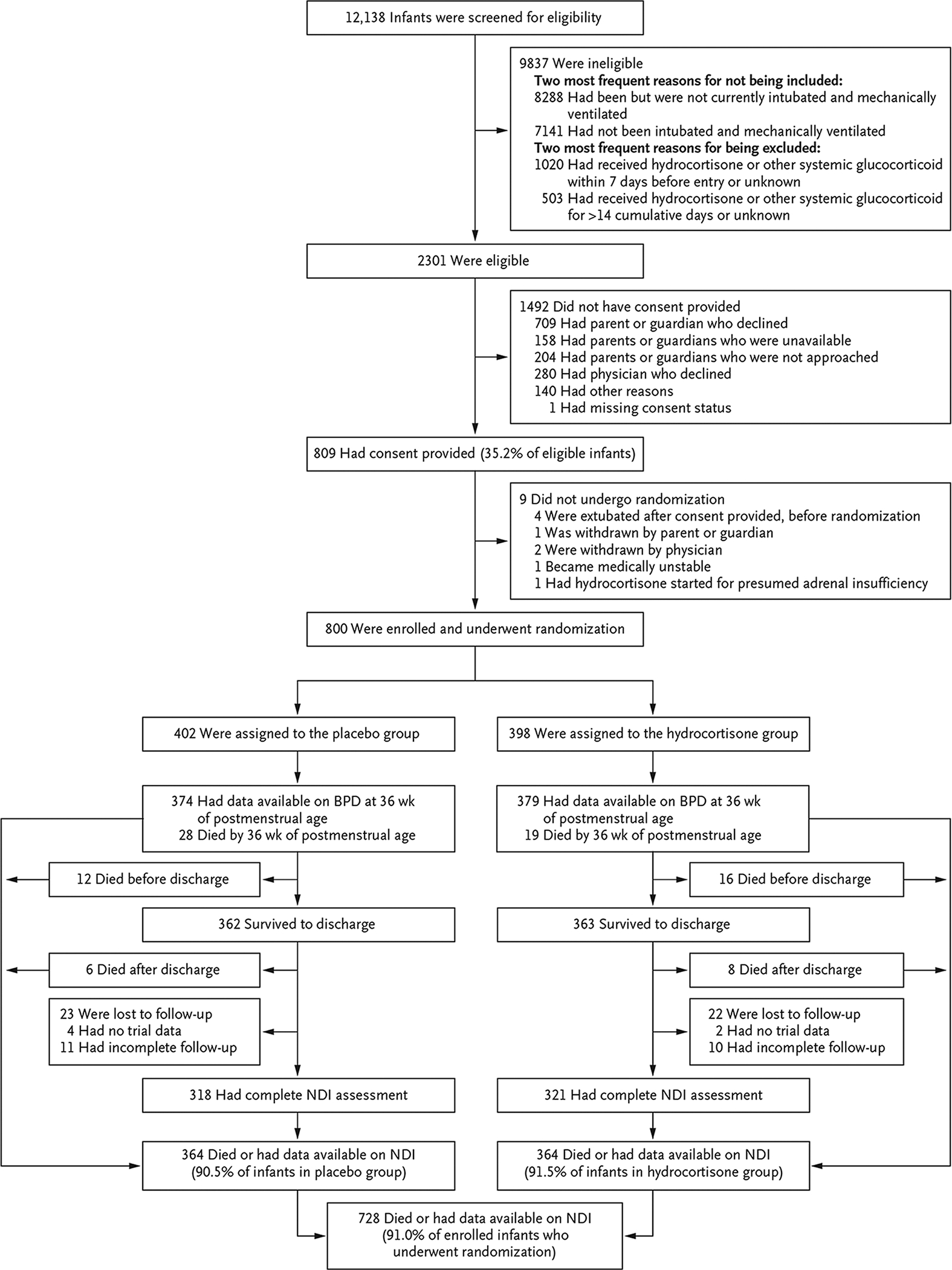
Infants were enrolled at 50 hospitals; however, the centers participating in the Neonatal Research Network changed over time, and the timing of approval by institutional review boards varied. Therefore, the number of hospitals enrolling at any given time was lower than 50. Although 1523 infants were excluded for previous receipt of glucocorticoids, only 339 of these infants had received glucocorticoid therapy for bronchopulmonary dysplasia (BPD). Thus, 3.4% of the 9837 ineligible infants were excluded for this reason. The majority (1184) had received early therapy for hypotension. There were 11 infants for whom the severity of neurodevelopmental impairment (NDI) could not be determined. According to the protocol for the follow-up study, from which we obtained the NDI data in our trial, NDI could be determined if a component of a binary indicator in the NDI definition was known as “Yes,” but the severity level could be determined only when other components of level of severity were not missing.
