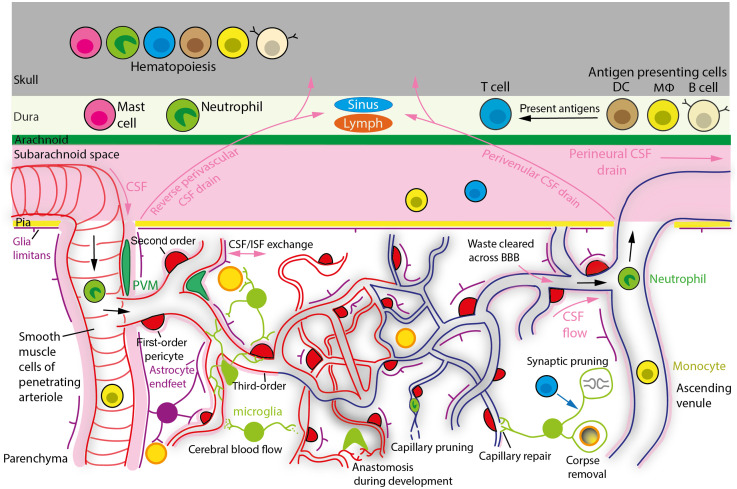
Fig. 1.
Organization of the brain vasculature, proposed waste clearance pathways and immune cells involved in brain surveillance. Blood in pial arteries enters the cerebral cortex parenchyma down PAs, goes through capillaries to supply oxygen and glucose to neurons, and exits the brain via AVs. Blood oxygenation decreases as it passes through the capillary bed, as indicated by the vessel color transitioning from red to blue. The PA is referred to as the zeroth-order vessel, the first branch off the arteriole as the first-order capillary, the branches of the first-order capillary as the second-order capillary and so on. Smooth muscle cells wrapping pial arteries and PAs, and pericytes with contractile processes around capillaries, control CBF by adjusting vessel diameter. CSF, which circulates in the subarachnoid space between the pia mater and arachnoid membrane, enters the perivascular space of PAs. Vessel pulsation driven by the heart, spontaneous vasomotion of SMCs and possibly first- to third-order capillary pericytes, creates a flow of CSF, which drains ISF waste either via reverse perivascular transport in small spaces of the PA wall, or through the brain via the glymphatic system (so called because AQP4 water channels in astroglial endfeet facilitate the exchange of CSF and ISF) possibly exiting via perivenular pathways to the subarachnoid space and dura. ISF might be drained (perineurally) along olfactory nerve rootlets into lymph vessels (not shown), via the dural meningeal lymphatics, across the BBB and/or venous sinuses into the blood and possibly through skull channels into the calvarial marrow. APCs (DCs, , and B cells) in the dura can capture CSF-derived brain antigens and present them to T cells, which modulate cognitive function by releasing neuromodulatory cytokines. The skull’s hematopoietic niche supplies leukocytes to the meninges. Cerebral waste molecules are also removed across ECs into the blood or phagocytosed by microglia and astrocytes. Microglia scan the brain for damage or infection related signals, prune synapses, at least in part by being programmed by T cells, regulate CBF, and NVC via purinergic signaling, repair damaged vessels by facilitating EC ligation and promote EC tip fusion during development, “anastomosis.” During aging, pericyte contraction and possibly neutrophil plugging (as occurs in AD) lead to a reduction of microvascular blood flow and may contribute to capillaries becoming pruned and phagocytosed by microglia.