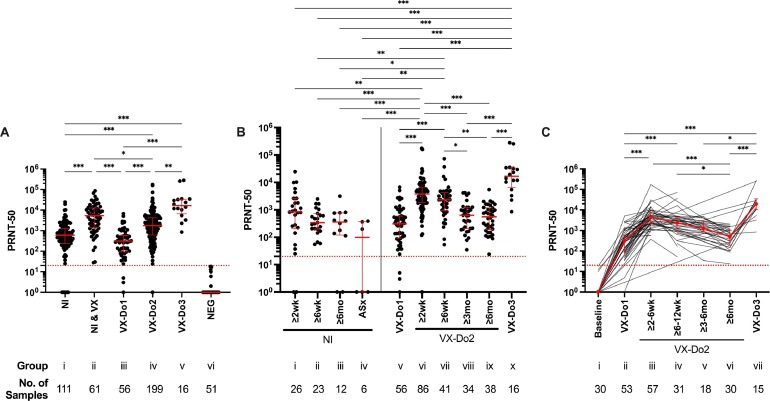
Fig. 1.
SARS-CoV-2 PRNT-50 antibody titers. (A) Group comparisons among, i) individuals with a history of SARS-CoV-2 natural infection prior to COVID-19 vaccination (NI); ii) individuals with a history of SARS-CoV-2 natural infection and ≥ 10 days following at least one dose of a COVID-19 vaccine (NI & VX); iii) individuals without a history of SARS-CoV-2 natural infection, ≥ 10 and ≤ 42 days following one dose of a mRNA COVID-19 vaccine (VX-Do1), iv) individuals without a history of SARS-CoV-2 natural infection, ≥ 10 days following two doses/complete COVID-19 vaccination (VX-Do2), v) individuals without a history of SARS-CoV-2 natural infection, ≥ 10 days following booster dose (VX-Do3); and vi) SARS-CoV-2 naïve individuals (NEG). (B) Longitudinal analysis among individuals with a history of SARS-CoV-2 natural infection (left) or COVID-19 vaccination without a history of SARS-CoV-2 natural infection (right). Intervals for the SARS-CoV-2 naturally infected individuals (left) were as follows: i) ≥ 10 days to 6 weeks (NI, ≥ 2wk), ii) ≥ 6 weeks to 6 months (NI, ≥ 6wk), iii) ≥ 6 months (NI, ≥ 6mo) following symptom onset and/or PCR positivity, and iv) asymptomatic SARS-CoV-2 natural infection (ASx). Intervals for the individuals without a history of SARS-CoV-2 natural infection following COVID-19 vaccination (right) were as follows: v) ≥ 10 and ≤ 42 days following one dose of a COVID-19 vaccine (VX-Do1), vi) ≥10 days to 6 weeks (VX-Do2, ≥ 2wk), vii) ≥ 6 weeks to 3 months (VX-Do2, ≥ 6wk), viii) ≥ 3 months to 6 months (VX-Do2, ≥ 3 mo), ix) ≥ 6 months (VX-Do2, ≥ 6 months) following two doses/complete COVID-19 vaccination; and x) with complete COVID-19 vaccination and ≥10 days following booster dose (VX-Do3), without a history of SARS-CoV-2 infection. (C) Longitudinal cohort analysis among the individuals following COVID-19 vaccination without a history of SARS-CoV-2 infection for which multiple blood draws (n = 64) were obtained (two blood draws/collection points, n = 16; three blood draws, n = 22; four blood draws, n = 8; five blood draws, n = 7; six blood draws, n = 8; seven blood draws, n = 3). Samples were collected at i) baseline, ii) ≥ 10 and ≤ 42 days following one dose of a mRNA COVID-19 vaccine (VX-Do1), iii) ≥10 days to 6 weeks (VX-Do2, ≥ 2wk), iv) ≥ 6 weeks to 3 months (VX-Do2, ≥ 6wk), v) ≥ 3 months to 6 months (VX-Do2, ≥ 3mo), vi) ≥ 6 months (VX-Do2, ≥ 6 months) following two doses/complete COVID-19 vaccination; or vii) after complete COVID-19 vaccination and ≥10 days following booster/third dose (VX-Do3). Kruskal-Wallis ANOVA test for non-parametric data was used to evaluate group differences. Dotted red line is the limit of detection of the neutralizing antibody titer (PRNT ≥ 20). *p≤0.033, **p≤0.002, ***p<0.001.