Abstract
Background
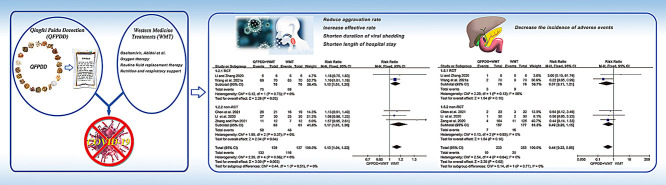
Qingfei Paidu decoction (QFPDD) showed to be beneficial for the treatment of coronavirus disease 2019 (COVID-19) in China.
Purpose
This study aimed to systematically assemble the evidence on the efficacy and safety of QFPDD combined with Western medicine treatments (WMT) for COVID-19.
Study design
Systematic review and meta-analysis.
Methods
A comprehensive literature search was conducted in PubMed, Embase, Cochrane Library, CNKI, CSTJ, CBM, Wanfang Data for clinical trials with a control arm until January 13, 2022. Studies matched the selection criteria were included. Data extraction and quality assessment of the included studies were independently conducted by two reviewers. Review Manager 5.4 was used for meta-analysis.
Results
A total of 9 trials including 1108 COVID-19 patients met the selection criteria. Meta-analysis demonstrated that QFPDD combined with WMT reduced aggravation rate (AR) by 71% [risk ratio (RR) = 0.29, 95% confidence intervals (CI) (0.17, 0.51)], increased effective rate (ER) by 13% [RR = 1.13, 95%CI (1.04, 1.22)], shortened 4.78 days of viral shedding [95%CI (-5.79, -3.77)] and 4.45 days of hospital stay [95%CI (-6.05, -2.86)], also decreased the incidence of adverse events (AE) by 56% [RR = 0.44, 95%CI (0.22, 0.89)].
Conclusion
QFPDD combined with WMT might reduce the proportion of severe cases and the incidence of AE, shorten the duration of viral shedding and length of hospital stay. More randomized controlled trials (RCTs) are required to confirm our findings in the future.
Keywords: Qingfei Paidu decoction, COVID-19, Western medicine treatments, Clinical effect, Systematic review, Meta-analysis
Abbreviations: AE, adverse events; AR, aggravation rate; CBM, China Biology Medicine; CI, confidence intervals; CNKI, China National Knowledge Infrastructure; COVID-19, coronavirus disease 2019; CSTJ, China Science and Technology Journal Database; ER, effective rate; MD, mean difference; MINORS, methodological index for non-randomized studies; NHC, the National Health Commission; non-RCTs, non-randomized concurrent trials; RR, risk ratio; PRISMA, the Preferred Reporting Items for Systematic Reviews and Meta-analyses; QFPDD, Qingfei Paidu decoction; RCTs, randomized controlled trials; SATCM, the State Administration of Traditional Chinese Medicine; TCM, traditional Chinese medicine; WMT, Western medicine treatments
Graphical abstract
.
Introduction
The coronavirus disease 2019 (COVID-19) pandemic is still threatening the global public health (Khanna et al., 2021; Ma et al., 2021). Despite intense scientific effort globally for finding specific drug for treatment of COVID-19 (Cao et al., 2020a; Hariyanto et al., 2021a; 2021b; 2021c; Komoda, 2021, Pan et al., 2020), unfortunately, previous studies demonstrated that some therapeutic agents including remdesivir, hydroxychloroquine, lopinavir/ritonavir, interferon and tocilizumab had not significant clinical effects on treating patients with COVID-19 (Cao et al., 2020a; Hariyanto et al., 2021b; Pan et al., 2020). However, traditional Chinese medicine (TCM) has achieved remarkable curative effect, playing a critical role in controlling COVID-19 (Cao et al., 2020b; Hu et al., 2020; Tian et al., 2020). Recent several meta-analysis studies demonstrated that TCM could improve clinical symptoms (including fever, cough, and fatigue, etc.) and lung lesions, shorten duration of fever and course of disease, and reduce the conversion rate from mild to severe (Fan et al., 2020; Liu et al., 2020; Xiong et al., 2020). Among them, Qingfei Paidu decoction (QFPDD) has shown notable therapeutic effects to treat COVID-19. Of 214 confirmed patients treated with QFPDD from four Chinese provinces between January 27 and February 5, 2020, more than 90% total effective rate (ER) was reached, among which more than 60% of patients presented noticeable improvement in symptoms according to data reported by the State Administration of Traditional Chinese Medicine (NHC, 2020a). QFPDD has been recommended as a general prescription in China (NHC, 2020b; SATCM, 2020).It has widely used all over the country, and achieved remarkable therapeutic effect (Liu et al., 2021a; Ren et al., 2020). QFPDD consists of four classic Chinese medicine prescriptions, i.e., Shegan Mahuang decoction, Maxing Shigan decoction, Xiaochaihu decoction, and Wuling powder, and contains 21 main herbal components (Table 1 ). Previous studies reported that QFPDD has good effectiveness on COVID-19 and can effectively prevent the worse disease progress, improve clinical symptoms and absorption of lung lesions, reduce death rates, while no serious adverse reactions have been reported (Chen et al.,2020b; Ji et al., 2021; Kang et al.,2020; Luo et al.,2021; Ma et al., 2020; Ren et al., 2020; Shi et al., 2020; Wang et al.,2021b; Zhang et al.,2021) . Notably, a study from South Korea showed that QFPDD was the most prescribed herbal medicine for COVID-19 in Korea in the first days of the disease and was the second most prescribed medicine across the whole course. In addition, COVID-19 related symptoms, including fever, dry cough, sore throat, and fatigue were also improved after treatment with QFPDD (Jang et al., 2021). QFPDD is suitable for mild, moderate, severe patients, and can be used reasonably in combination with other drugs for critical patients (NHC, 2020b). Thus, we aimed to systematically assemble the evidence on the efficacy and safety of QFPDD in combination with Western medicine treatment (WMT) for COVID-19 treatment, and provide reference for clinicians in clinical practice.
Table 1.
Components of Qingfei Paidu decoction.
| Chinese name(Pinyin) | English name | Latin name | Dose(gram) |
|---|---|---|---|
| Bai Zhu | Largehead Atractylodes Rhizome | Atractylodis Macrocephalae Rhizoma | 9 |
| Chai Hu | Chinese Thorawax Root | Bupleuri Radix | 16 |
| Chen Pi | Tangerine Peel | Citri Reticulatae Pericarpium | 6 |
| Fu Ling | Indian BueadTuckahoe | Poria | 15 |
| Gui Zhi | Cassiabarktree Twig | Cinnamomi Ramulus | 9 |
| Huang Qin | Baikal Skullcap Root | Scutellariae Radix | 6 |
| Huo Xiang | Wrinkled Gianthyssop Herb | Agastache rugosus | 9 |
| Jiang Ban Xia | Ternate Pinellia | Pinelliae Rhizoma | 9 |
| Kuan Dong Hua | Common Coltsfoot Flower | Farfarae Flos | 9 |
| Ma Huang | Chinese Ephedrs Herb | Ephedrae Herba | 9 |
| Shan Yao | Common Yan Rhizome | Dioscoreae Rhizoma | 12 |
| She Gan | Blackberrglily Rhizome | Belamcandae Rhizoma | 9 |
| Sheng Jiang | Fresh Ginger | Zingiberis Rhizoma Recens | 15 |
| Sheng Shi Gaoa | plaster stone | Raw Gypsum | 15∼30* |
| Xing Ren | Ansu Apricot Seed | Armeniacae Semen Amarum | 9 |
| Xi Xin | Manchurian Wildginger Herb | Asari Radix et Rhizoma | 6 |
| Ze Xie | Oriental Waterplantain Tuber | Alismatis Rhizoma | 9 |
| Zhi Gan Cao | Liquorice Root | Glycyrrhizae Radix et Rhizoma | 6 |
| Zhi Shi | Immature Bitter Orange | Fructus Aurantii Immaturus | 6 |
| Zhu Ling | Agaric | Polyporus | 9 |
| Zi Wan | Tatarian Aster Root and Rhizome | Asteris Radix et Rhizoma | 9 |
Note: Cook in advance 30 min
If the patient does not have a fever, the amount of gypsum should be little. If having a fever or an attack of fever, the amount of gypsum can be increased.
Methods
This study was conducted following the Preferred Reporting Items for Systematic Reviews and Meta-analyses (PRISMA) (Moher et al., 2009).
Search strategy
Two reviewers (S.H.L. and L.T.) independently searched PubMed, Embase, Cochrane Library by using English, China National Knowledge Infrastructure (CNKI), China Science and Technology Journal Database (CSTJ), China Biology Medicine (CBM), and Wanfang Data by using Chinese, following search terms: (“COVID-19” OR “2019-nCoV” OR “SARS-CoV-2” OR “the Novel Coronavirus Pneumonia” OR “NPC”) AND (“Qingfei Paidu decoction” OR “QPD” OR “QFPDD” OR “Lung Cleansing and Detoxifying Decoction”) AND (“clinical trial” OR “randomized controlled trial” OR “randomised controlled trial” or “random” or “trial “or “RCT”) from the inception date to January 13, 2022. References of important articles were searched manually for possible relevant studies.
Study selection
Type of studies
We included all types of clinical studies, including randomized controlled trials (RCTs), and non-randomized concurrent trials (non-RCTs).
Type of participants
Trials were considered eligible for inclusion if they were conducted in COVID-19 patients confirmed by “Diagnosis and Treatment Protocol for COVID-19” issued by the National Health Commission, participants enrolled were treated with QFPDD for at least a course (three days), in addition, without restrictions on age, gender, or nationality in the present study.
Type of interventions
We included studies in which patients were given QFPDD combined with WMT in comparison to patients who were treated with WMT alone. However, trials that involved acupuncture, moxibustion, and massage were excluded.
According to the “Diagnosis and Treatment Protocol for Novel Coronavirus Pneumonia Diagnosis and Treatment Protocol for COVID-19” released by National Health Commission & State Administration of Traditional Chinese Medicine, WMT is a therapy that comprises antivirals, antibiotics and/or other supportive treatments such as oxygen therapy, used in Western medicine. Details are shown in Supplementary Table 1.
Prescription forms of QFPDD
In our study, prescription forms of QFPDD were taken from the “Diagnosis and Treatment Protocol for Novel Coronavirus Pneumonia Diagnosis and Treatment Protocol for COVID-19” (NHC, 2020b). The detailed components and doses of QFPDD are shown in Table 1.
All studies in this review used a uniform way to administrate QFPDD, as follows: after admission, patients enrolled in each study received QFPDD, with/without an antibiotic belonging to WMT. The treatments were given twice a day (one in the morning and one in the evening), and three days a course, with a total duration of 3 days-2 weeks with an average of 10 days.
Type of outcome measures
The primary outcomes included Aggravation rate (AR) and Effective rate (ER).
AR refers to the percentage of aggravation. Aggravation was defined to meet one of the following conditions; a) patients with exacerbations during the treatment and turned to severe or critical at least one grade of clinical classification progression of the COVID-19 Treatment Protocol (6th or 7th Edition); b) Hospital transfer was considered as severe or critical COVID-19; c) Transfer to ICU; d) Respiratory failure requiring mechanical ventilation, ECMO, high-flow nasal catheter oxygen therapy, or noninvasive ventilation; e) Shock; f) Death.
ER was defined as the ratio of number of people cured or in remission per group to the total number of people in that group.
The word ‘cured’ referred to having fully recovered and being discharged from the hospitals, while “remission” meant that symptoms, signs, and laboratory indicators were improved in varying degrees. The remission of symptoms must conform to one of following conditions, a) Body temperature returned to normal for more than 1 day: temperature < 37.3 °C (under the armpit) or mouth temperature ≤ 37.5 °C, or anal or ear temperature ≤ 37.8 °C; b) Stop coughing for more than 1 day; c) Stop dry coughing for more than 1 day; d) Fatigue disappeared for more than 1 day; e) Anorexia returned to normal for more than 1 day; f) Sore throat disappeared for more than 1 day.
Secondary outcomes: duration of viral shedding, length of hospital stay and the adverse events (AE) during hospitalizations.
In addition, studies with unavailable full text or incomplete data or similar paper previously published, were excluded from this review.
Data extraction
The titles and abstracts of potentially eligible publications were independently screened by two reviewers (L.T and S.H.L) according to the search strategy. Then, full texts of the possible studies were retrieved and reviewed based on the inclusion and exclusion criteria. The process of study selection was documented using a PRISMA flow diagram (Moher et al., 2009). Data of the included trials were extracted independently by two reviewers (H.J.G and X.Y.J). The following information was retrieved: first author's name, publication year, study design, original place of patients, sample size, severity of disease, mean participant age, sex distribution, complications, diagnostic criteria, interventions in the treatment and control groups, duration, and outcome measures. Any discrepancy was discussed with the third reviewer (N.N.S).
Methodological quality assessment
The methodological quality of trials was graded by two reviewers (N.L. and R.B.C.) independently. RCTs were evaluated according to the Cochrane collaboration risk of bias tool (Higgins et al., 2020), while non-randomized experimental studies were evaluated by methodological index for non-randomized studies (MINORS) (Slim et al., 2003; Zeng et al., 2012).
Data synthesis and analysis
This meta-analysis was conducted using Review Manager 5.4 provided by the Cochrane Collaboration (Higgins et al., 2020). For dichotomous variables, the risk ratio (RR) with 95% confidence intervals (CI) were presented. For continuous variables, the weighted mean difference (MD) with 95% CI were presented. I2 statistic were used to quantify the size of heterogeneity among the studies. An I2 ≤ 50% indicated a low heterogeneity among studies, and fixed effects model was used to synthesize the estimates, otherwise random effects model was used (Higgins et al., 2003; Ioannidis et al., 2007). In addition, according to the type of studies, we performed subgroup analysis to minimize the heterogeneity.
Results
Study identification
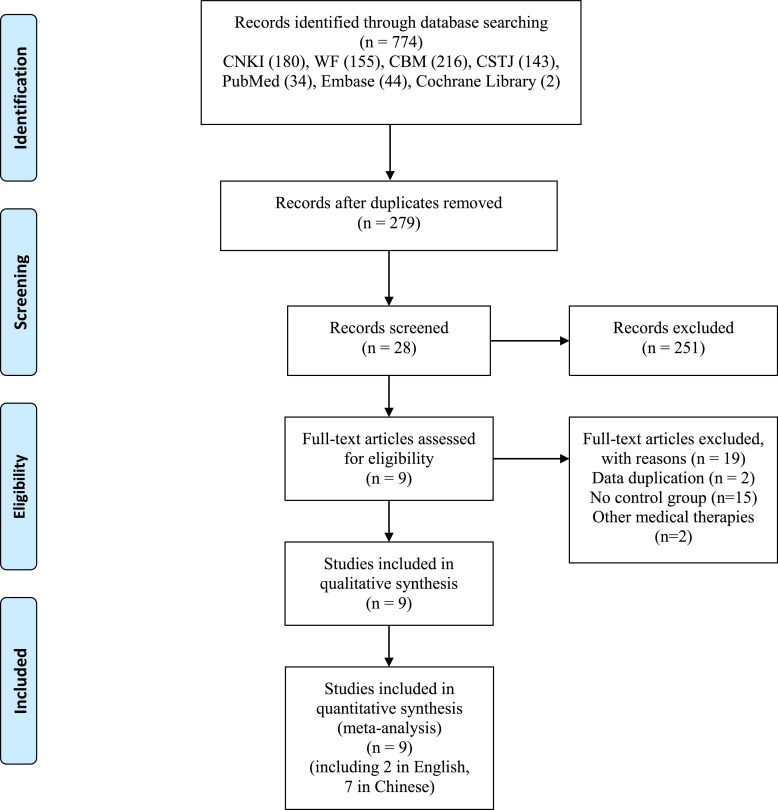
According to the search strategy, 774 potentially relevant publications were searched by both electronic and manual searching approaches. After eliminating duplicates, the titles, abstracts and full text of 279 records were screened. Finally, we included 9 studies with 1108 COVID-19 patients (Chen et al., 2021; Li et al., 2020; Li and Zhang, 2020; Liu et al., 2021b; Wang et al., 2021a; Xin et al., 2020; Yu et al., 2020b; Zeng et al., 2020; Zhang and Pan, 2021). WMT in each study were shown in Supplementary Table 1. The screening process was shown in Figure 1 . Seven papers were published in Chinese and two in English (Liu et al., 2021b; Xin et al., 2020).
Fig. 1.
PRISMA flow diagram of study selection process.
Characteristics of included studies
Nine studies included were all conducted in China. Two were RCTs (Li and Zhang, 2020; Wang et al., 2021a) and the rest were non-RCTs (Chen et al., 2021; Li et al., 2020; Liu et al., 2021b; Xin et al., 2020; Yu et al., 2020b; Zeng et al., 2020; Zhang and Pan, 2021). One study was conducted in several provinces of China (Chen et al., 2021). Six studies were conducted in Hubei province (Li et al., 2020; Liu et al., 2021b; Wang et al., 2021a; Xin et al., 2020; Yu et al., 2020b; Zhang and Pan, 2021), one in Beijing (Zeng et al., 2020), and one in Shanxi province (Li and Zhang, 2020). The sample size ranged from 12 to 446 participants. Among them, 37 non-severe patients were reported in Chen et al. (2021); 55 non-severe patients in Li et al. (2020), 31 moderate patients in Yu et al. (2020b); all patients were severe cases in two studies (Li and Zhang, 2020; Zhang and Pan, 2021). Five studies reported patients had got complications (Chen et al., 2021; Li et al., 2020; Liu et al., 2021b; Xin et al., 2020; Yu et al., 2020b). All research interventions were QFPDD combined with WMT, the course of treatment was no less than 3 days in all studies. Main characteristics were shown in Table 2 .
Table 2.
Characteristics of the 9 studies included in the meta-analysis.
| Study | Study design | Study sites | Sample size (T/C) | Disease severity | Gender | Age (years) | Comorbidities | Diagnostic criteria | Intervention | Duration | Outcome measures | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| T | C | T (M/F) | C (M/F) | T/C | T | C | T (QFPDD +WMT) | C(WMT) | |||||||
| Li et al. 2020 | Non-RCT | Hubei | 30/30 | Severe (3), Non- Severe (27) |
Severe (2), Non- Severe (28) |
15/15 | 13/17 | 53.60 ± 0.26/ 50.43 ± 0.34 |
NR | NR | Version 6 | QFPDD + WMT | Oseltamivir, Abby dole, Lopinavir/ritonavir, no specific antibacterial, others | NR | AR, ER, LHS, AE |
| Li and Zhang. 2020 | RCT | Shanxi | 6/6 | Severe (6) | Severe (6) | 3/3 | 2/4 | 50.00 ± 10.00/ 52.00 ± 6.56 |
None None | Version 7 | QFPDD + WMT | Alpha interferon, Ribavirin, no specific antibacterial, others | NR | ER, LHS, AE | |
| Xin et al.2020 | Non-RCT | Hubei | 37/26 | Moderate (37) | Mild (24), Moderate (2) | 17/20 | 12/14 | 23.5 – 89.9/ 15.3 – 81.9 |
DM (4), HBP (7), CAD (4) | DM (3), HBP (9), CAD (1) | Version 6 | QFPDD + WMT | Interferon, Abby dole, Lopinavir, No specific antibacterial, others | 6d | AR, LHS |
| Yu et al. 2020b | Non-RCT | Hubei | 43/46 | Severe (29), Moderate (14) | Severe (29), Moderate (17) | 22/21 | 28/18 | 64.23 ± 2.51/ 60.50 ± 2.08 |
HBP (5), DM (5), CAD (3) | HBP (5), DM (4), CAD (2) | Version 6 | QFPDD + WMT | No specific drugs were specified, others | 10-15d | DVS, LHS |
| Zeng et al.2020 | Non-RCT | Beijing | 104/125 | Moderate (104) | Moderate (125) | 56/48 | 69/57 | 46.65 ± 6.21/ 46.21 ± 5.62 |
None None | Version 7 | QFPDD + WMT | Lopinavir, No specific antibacterial, others | NR | DVS, LHS, AE | |
| Chen et al. 2021 | Non-RCT | Sichuan, Heilongjiang, Shanxi, Fujian, Guangxi, Chongqing, Hebei | 23/22 | Severe (4), Non- Severe (19) |
Severe (4), Non- Severe (18) |
19/4 | 13/9 | 47.0 (40.0, 54.0) /43.5 (33.0, 48.5) | Hepatitis b (all), HBP and DM (10) | Hepatitis b (all), HBP and DM (8) | Version 3, 4, 5, 6 | QFPDD + WMT | Arbidol, Lopinavir/Ritonavir, Ribavirin, Moxifloxaci, Cephalosporins, others | 6d | ER, DVS, LHS |
| Liu et al.2021 | Non-RCT | Hubei | 223/223 | NR | NR | 111/112 | 113/110 | 59.0 (51.0 - 66.0)/ 61.0 (49.0 – 69.0) |
HBP (43), DM (17), CAD (11), COPD (1), Cancer (6), CVD (5) | HBP (39), DM (10), CAD (14), COPD (1), Cancer (6), CVD (5) | Version 6 | QFPDD + WMT | Version 6 | 3d at least | AR |
| Wang et al.2021a | RCT | Hubei | 70/70 | Moderate (70) | Moderate (70) | 35/35 | 36/34 | 48 ± 13.2/ 49 ± 13.3 |
None None | Version 6 | QFPDD + WMT | Abby dole, Moxifloxacin, others | 10d | ER, LHS, AE | |
| Zhang and Pan.2021 | Non-RCT | Hubei | 12/12 | Severe (12) | Severe (12) | 6/6 | 7/5 | 61.42 ± 13.24/ 62.25 ± 14.69 |
NR NR | Version 7 | QFPDD + WMT | Abby dole, Alpha interferon, Moxifloxacin, others | 7d | AR, ER | |
Abbreviations: AR: aggravation rate; AE: adverse events; C: control group; CAD: coronary artery disease; CKD: chronic kidney disease; COPD: chronic obstructive pulmonary disease; CVD: cerebrovascular disease; DM: diabetes mellitus; DVS: duration of viral shedding; Emp: emphysema; ER: effective rate; F: female; HBP: high blood pressure; LHS: length of hospital stay; M: male; NR: no reported; non-RCT: non-randomized concurrent trial; QFPDD: Qingfei Paidu decoction; RCT: randomized controlled trial; T: treatment group; Version 3: Diagnosis and Treatment Protocol for Novel Coronavirus Pneumonia (Trial Version 3); Version 4: Diagnosis and Treatment Protocol for Novel Coronavirus Pneumonia (Trial Version 4); Version 5: Diagnosis and Treatment Protocol for Novel Coronavirus Pneumonia (Trial Version 5); Version 6: Diagnosis and Treatment Protocol for Novel Coronavirus Pneumonia (Trial Version 6); Version 7: Diagnosis and Treatment Protocol for Novel Coronavirus Pneumonia (Trial Version 7); WMT: Western medicine treatments. In this review, severe patients include severe and critical patients, non-severe patients include mild and moderate patients.
Assessment of methodological quality
Among the nine studies included, two were RCTs (Li and Zhang, 2020; Wang et al., 2021a) and the other seven were non-RCTs. Both the two RCTs did not report the method of generating random sequences, and none of them reported the use of allocation concealment and blinding. However, they reported outcomes completely and no reported outcome selectively. All seven non-RCTs scored fifteen for the risk of bias, and were summarized in Table 3 . In summary, we considered the overall risk of bias of the nine included studies was moderate.
Table 3.
Risk of biases of including non-RCTs by MINORS.
| Items | Li et al. (2020) | Xin et al. (2020) | Yu et al. (2020b) | Zeng et al. (2020) | Chen et al. (2021) | Liu et al. (2021) | Zhang and Pan (2021) |
|---|---|---|---|---|---|---|---|
| A clearly stated aim | 2 | 2 | 2 | 2 | 2 | 2 | 2 |
| Inclusion of consecutive patients | 1 | 1 | 1 | 1 | 1 | 1 | 1 |
| Prospective collection of data | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Endpoints appropriate to the aim of the study | 2 | 2 | 2 | 2 | 2 | 2 | 2 |
| Unbiased assessment of the study endpoint | 2 | 2 | 2 | 2 | 2 | 2 | 2 |
| Follow-up period appropriate to the aim of the study | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Loss to follow up less than 5% | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Prospective calculation of the study size | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| An adequate control group | 2 | 2 | 2 | 2 | 2 | 2 | 2 |
| Contemporary groups | 2 | 2 | 2 | 2 | 2 | 2 | 2 |
| Baseline equivalence of groups | 2 | 2 | 2 | 2 | 2 | 2 | 2 |
| Adequate statistical analyses | 2 | 2 | 2 | 2 | 2 | 2 | 2 |
| Total | 15 | 15 | 15 | 15 | 15 | 15 | 15 |
Primary outcomes
ER assessment
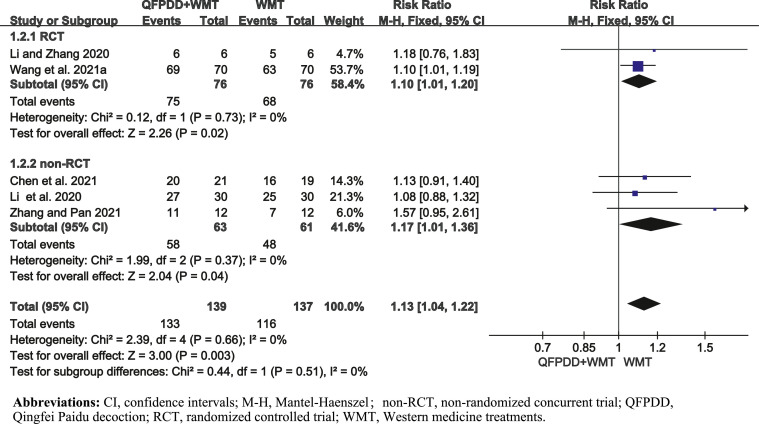
Five studies (Chen et al., 2021; Li et al., 2020; Li and Zhang, 2020; Wang et al., 2021a; Zhang and Pan, 2021) reported ER, involving 276 patients. We performed subgroup analysis according to different study designs. The results showed that the ER in QFPDD combined with WMT group was better than WMT group alone by 13% [pooled RR = 1.13, 95%CI (1.04, 1.22)] (Fig. 2 ). There was no heterogeneity.
Fig. 2.
Forest plot of QFPDD combined with WMT on ER in COVID-19 patients.
AR assessment
Four studies (Li et al., 2020; Liu et al., 2021b; Xin et al., 2020; Zhang and Pan, 2021) reported the AR involving 593 patients. The results demonstrated that the AR in QFPDD combined with WMT group was lower than that the WMT group by 71% [pooled RR = 0.29, 95%CI (0.17, 0.51)] (Supplementary Figure 1).
Secondary outcomes
Duration of viral shedding (days)
Two studies (Yu et al., 2020b; Zeng et al., 2020) reported the duration of viral shedding, involving 318 patients. The analysis results showed that QFPDD combined with WMT group shortened 4.78 days of viral shedding compared to WMT group alone [pooled MD = -4.78, 95%CI (-5.79, -3.77)] (Supplementary Figure 2).
Length of hospital stay (days)
Seven studies reported the length of hospital stay, of which, two studies (Chen et al., 2021; Xin et al., 2020) reported median. The rest five studies reported mean and standard deviation (Li et al., 2020; Li and Zhang, 2020; Wang et al., 2021a; Yu et al., 2020b; Zeng et al., 2020), involved 378 patients. QFPDD combined with WMT could shorten 4.45 days of hospital stay, compared with WMT alone [pooled MD = -4.45, 95%CI (-6.05, -2.86)] (Supplementary Figure 3).
We also analyzed the length of hospital stay on account of poor homogeneity based on disease severity (p < 0.00001, I2 = 98%), and presence or absence of comorbidities (p < 0.00001, I2 = 98%).
AE assessments
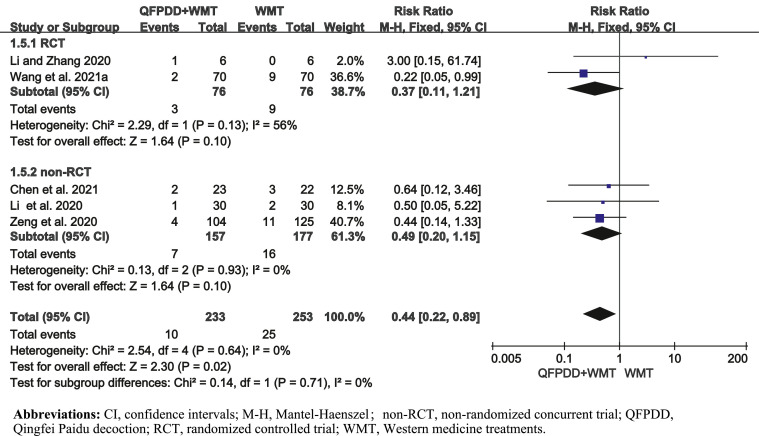
Five studies (Chen et al., 2021; Li et al., 2020; Li and Zhang, 2020; Wang et al., 2021a; Zeng et al., 2020) reported AE, involving 486 patients. The results showed that QFPDD combined with WMT decreased the occurrence of AE by 56% compared with WMT alone [pooled RR = 0.44, 95%CI (0.22, 0.89)] (Fig. 3 ). AE mainly reflected as nausea, diarrhea, pruritus, other acute respiratory illnesses, and shock.
Fig. 3.
Forest plot of QFPDD combined with WMT on AE in COVID-19 patients.
Sensitivity analyses
We further performed sensitivity analyses using RevMan 5.4 for AR and ER, and the results were not significantly difference in the primary analysis (Supplementary Tables 2-3). The sensitivity analysis for AR and ER were shown in Supplementary Figures 4-5.
Discussion
This meta-analysis showed that QFPDD combined with WMT was more effective in treating COVID-19 than WMT alone. The combination reduced the AR, increased the ER, shortened the duration of viral shedding and the length of hospital stay.
TCM therapy has been used to treat diseases for more than 5,000 years in China (Li and Kan, 2017). Additionally, TCM has a long history in prevention and treatment of infectious diseases (Ren et al., 2020; Yue, 2012). Xu et al. (2020) reported that QFPDD when used alone could regulate the body immunity, inhibit inflammatory response and reduce lung injury based on pharmacology (Xu et al., 2020). Chen et al. (2020a) and Zhong et al. (2020) found QFPDD had a protection effect on COVID-19 by regulating a complex molecular network with safety and efficacy. Part of the mechanism was associated with the regulation of antiviral, anti-inflammatory activity and metabolic programming (Chen et al., 2020a; Zhong et al. 2020). Cao et al. (2020b) and Zhao et al. (2020) demonstrated that QFPDD could exhibit immune regulation, anti-infection, anti-inflammation, and multi-organ protection (Cao et al., 2020b; Zhao et al., 2020).Therefore, QFPDD combined with WMT could improve effectively clinical ER, improve the clinical symptoms and prevent COVID-19 disease based on above reason.
A total of 9 papers were included in our study, the results showed that QFPDD combined with WMT improved clinical ER effectively (Chen et al., 2021; Li et al., 2020; Li and Zhang, 2020; Wang et al., 2021a; Zhang and Pan., 2021), in line with the reports from Jang et al. (2021), Ni et al.(2020), Shi et al. (2020), Sun et al. (2021), Wang et al. (2020), and Zhong et al. (2020). QFPDD combined with WMT shortened duration of viral shedding (Yu et al., 2020b; Zeng et al., 2020) and length of hospital stay (Li et al., 2020; Li and Zhang, 2020; Wang et al., 2021a; Yu et al., 2020b; Zeng et al., 2020), which is consistent with the studies published (Sun et al.,2021; Yu et al.,2020a). Moreover, QFPDD combined with WMT reduced the AR (Li et al., 2020; Liu et al. 2021b; Xin et al., 2021; Zhang and Pan., 2021), which had been proved in previous studies (Ma et al., 2020; Yu et al.,2020a). In addition, Zhang et al (2021) found that QFPDD was associated with a substantially lower risk of in-hospital mortality among hospitalized COVID-19 patients. Additionally, incidence rate of AE was less in QFPDD group (Chen et al. 2021; Li et al., 2020; Li and Zhang, 2020; Wang et al., 2021a; 2021b; Zeng et al., 2020), implying no serious AE related to QFPDD were found. Chen et al. (2021) and Kageyama et al. (2021) demonstrated that no serious AE were observed. Despite most above studies observed that QFPDD combined with WMT improved effective rate and had favorable treatment outcome; Xin et al. (2020) found that neither mortality nor length of hospitalization was affected.
Notably, Wang et al. (2021b) reported a systematic review and meta-analysis on efficacy and safety of QFPDD for treating COVID-19. They observed that QFPDD combined with WMT might decrease the time for nucleic acid conversion, shorten the length of hospital stay, shorten the duration of symptoms recovery of fever, cough and chest CT, improve the overall TCM symptom scores, and change the laboratory indexes. Despite 16 studies were enrolled, seven of them were pre–post studies and one study had no control group. Thus, the conclusions of that review might have more bias.
Conclusion and perspectives
There were several limitations in the present Review. First, all studies involved were conducted in China, thus, we lacked data on QFPDD use outside China. Second, among the nine studies involved, seven were in Chinese and two were in English. There might be potential publication bias. Despite above limitations, our study also has some strengths. The present study assessed efficacy and safety of QFPDD combined with WMT for the treatment of COVID-19 comprehensively and systematically. Moreover, QFPDD combined with WMT for COVID-19 presented favorable outcome, which can provide reference for doctors in clinical practice.
In summary, QFPDD combined with WMT reduces the AR, improves the clinical efficacy, shortens the duration of viral shedding and the length of hospital stay, and decreases AE. Further multi-center RCTs on efficacy and safety of QFPDD is necessary to be conducted in and outside China to confirm our findings.
Authors contributions
Y.Y.W., Y.P.W., H.M.Z., Y.M., and L.Z. designed the study. L.Z., Y.M., and N.L. carried out the statistical analysis, drew the tables and pictures. L.Z., Y.M., N.N.S., S.H.L., and L.T. drafted the manuscript, L.Z. conducted data-analysis and drafted the manuscript; S.H.L., L.T., X.Y.J., R.B.C., Y.P.F., N. L., H.J.G, G.K.C, and Y.M. searched the literature, collected the data, and assessed the methodological quality of included studies; X.Y.J., Y.W.G., and W.W. assisted with the design of PICOs and interpreted the results. Y.Y.W., Y.P.W., H.M.Z., Y.M., and L.Z. conceived the study and over sought the study implementation and provided the methodological guidance, conceived the study, designed the PICOs and interpreted the study results. All authors read and approved the final manuscript.
Funding
This study was supported by CACMS Innovation Fund (CI2021A00704), the National Key Research and Development Project (2018YFC1704401), National Natural Science Foundation of China (82105055), “COVID-19 Project of National Administration of Traditional Chinese Medicine(2020ZYLCYJ07-1), and COVID-19 project of National Administration of Traditional Chinese Medicine (GZY-KJS-2021-007).
Declaration of Competing Interest
The authors declare that they have no competing interests.
Acknowledgments
We would like to thank all the members in the present study, and we also thank Prof. Shengjie Lai from University of Southampton and Prof. Dongshan Zhu from University of Shandong for language editing of the manuscript.
Footnotes
Supplementary material associated with this article can be found, in the online version, at doi:10.1016/j.phymed.2022.154166.
Appendix. Supplementary materials
References
- Cao B., Wang Y., Wen D., Liu W., Wang J., Fan G., Ruan L., Song B., Cai Y., Wei M., Li X., Xia J., Chen N., Xiang J., Yu T., Bai T., Xie X., Zhang L., Li C., Yuan Y., Chen H., Li H., Huang H., Tu S., Gong F., Liu Y., Wei Y., Dong C., Zhou F., Gu X., Xu J., Liu Z., Zhang Y., Li H., Shang L., Wang K., Li K., Zhou X., Dong X., Qu Z., Lu S., Hu X., Ruan S., Luo S., Wu J., Peng L., Cheng F., Pan L., Zou J., Jia C., Wang J., Liu X., Wang S., Wu X., Ge Q., He J., Zhan H., Qiu F., Guo L., Huang C., Jaki T., Hayden F.G., Horby P.W., Zhang D., Wang C. A trial of lopinavir-ritonavir in adults hospitalized with severe COVID-19. N. Engl. J. Med. 2020;382:1787–1799. doi: 10.1056/NEJMoa2001282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao P., Wu S., Wu T., Deng Y., Zhang Q., Wang K., Zhang Y. The important role of polysaccharides from a traditional Chinese medicine-lung cleansing and detoxifying decoction against the COVID-19 pandemic. Carbohydr. Polym. 2020;240 doi: 10.1016/j.carbpol.2020.116346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen J., Wang Y.K., Gao Y., Hu L.S., Yang J.W., Wang J.R., Sun W.J., Liang Z.Q., Cao Y.M., Cao Y.B. Protection against COVID-19 injury by Qingfei Paidu Decoction via anti-viral, anti-inflammatory activity and metabolic programming. Biomed. Pharmacother. 2020;129 doi: 10.1016/j.biopha.2020.110281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen H., Song D., Gao T., Yang J., Deng Q. Qingfei Paidu Decoction”—A possible choice of phytotherapy for severe acute respiratory infection caused by coronavirus disease-19. Am. J. Plant. Sci. 2020;11:1111–1136. doi: 10.4236/ajps.2020.117079. [DOI] [Google Scholar]
- Chen R.B., Shi N.N., Li H.Z., Jiao L.W., Ma Y., Liu B., Xiong Y.B., Luan X.J., Wu G.H., Li J.K., Zhang X.Y., Zhu J.T., Xiao F.R., Wang J.Y., Li Q.T., Lei X., Huang J., Huang Q.H., Wang Y.D., Wang X.Y., Xiao Y.H., Liu H.D., Liu J.P., Hou Y.L., Zhang L.S., Xie H.J., Bai W.G., Zhang H.M., Wang Y.P., Wang Y.Y. Qingfei Paidu Decoction combined with Western medicine routine treatment for coronavirus disease 2019 (COVID-19) concomitant with chronic hepatitis b: A multi-center retrospective study. J. Tradit. Chin. Med. 2021;62:1694–1700. doi: 10.13288/j.11-2166/r.2021.19.008. Chinese. [DOI] [Google Scholar]
- Fan A.Y., Gu S., Alemi S.F. Chinese herbal medicine for COVID-19: Current evidence with systematic review and meta-analysis. J. Integr. Med. 2020;18:385–394. doi: 10.1016/j.joim.2020.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Higgins J.P., Thompson S.G., Deeks J.J., Altman D.G. Measuring inconsistency in meta-analyses. BMJ. 2003;327:557–560. doi: 10.1136/bmj.327.7414.557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Higgins J.P., Thomas J., Chandler J., Cumpston M., Li T., Page M.J. Cochrane handbook for systematic reviews of interventions version 6.1 (updated september 2020) Cochrane. 2020;2020 www.training.cochrane.org/handbook Available from. [Google Scholar]
- Hu K., Guan W.J., Bi Y., Zhang W., Li L., Zhang B., Liu Q., Song Y., Li X., Duan Z., Zheng Q., Yang Z., Liang J., Han M., Ruan L., Wu C., Zhang Y., Jia Z.H., Zhong N.S. Efficacy and safety of lianhuaqingwen capsules, a repurposed Chinese herb, in patients with coronavirus disease 2019: A multicenter, prospective, randomized controlled trial. Phytomedicine. 2020;85 doi: 10.1016/j.phymed.2020.153242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hariyanto T.I., Halim D.A., Rosalind J., Gunawan C., Kurniawan A. Ivermectin and outcomes from COVID-19 pneumonia: A systematic review and meta-analysis of randomized clinical trial studies. Rev. Med. Virol. 2021;32:e2265. doi: 10.1002/rmv.2265. [DOI] [Google Scholar]
- Hariyanto T.I., Hardyson W., Kurniawan A. Efficacy and safety of tocilizumab for coronavirus disease 2019 (COVID-19) Patients: A systematic review and meta-analysis. Drug. Res. 2021;71:265–274. doi: 10.1055/a-1336-2371. [DOI] [PubMed] [Google Scholar]
- Hariyanto T.I., Kurniawan A. Tocilizumab administration is associated with the reduction in biomarkers of coronavirus disease 2019 infection. J. Med. Virol. 2021;93:1832–1836. doi: 10.1002/jmv.26698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ioannidis J.P., Patsopoulos N.A., Evangelou E. Heterogeneity in meta-analyses of genome-wide association investigations. PLoS One. 2007;2:e841. doi: 10.1371/journal.pone.0000841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jang S., Kim D., Yi E., Choi G., Song M., Lee E.K. Telemedicine and the use of Korean medicine for patients with COVID-19 in South Korea: Observational study. JMIR Public Health Surveill. 2021;7:e20236. doi: 10.2196/20236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ji X.Y., Ma Y., Shi N.N., Liang N., Chen R.B., Liu S.H., Shi S., Wu G.H., Li J.K., Chen H., Wang J.W., Na H., Zhou Y.C., Li M.Q., Wang Y.D., Hu X.M., Hu Y.H., Liu Z., Xie H.J., Zhang L.S., Zhang H.M., Wang Y.P., Wang Y.Y. Clinical characteristics and treatment outcome of COVID-19 patients with stroke in China: A multicenter retrospective study. Phytomedicine. 2021;81 doi: 10.1016/j.phymed.2020.153433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang B.H., Choi Y.K., Jeon C.Y., Yang S.B. The effects of Qingfei Paidu Decoction on coronavirus disease-19: A narrative review. J. Inter. Kor. Med. 2020;41:424–433. doi: 10.22246/jikm.2020.41.3.424. Korean. [DOI] [Google Scholar]
- Kageyama Y., Aida K., Kawauchi K., Morimoto M., Ebisui T., Akiyama T., Nakamura T. Qingfei Paidu Decoction, a Chinese herbal medicine against COVID‑19, elevates the blood levels of pro‑inflammatory cytokines: An open‑label, single‑arm pilot study. WASJ. 2021;3:1–7. doi: 10.3892/wasj.2021.96. [DOI] [Google Scholar]
- Khanna K., Kohli S.K., Kaur R., Bhardwaj A., Bhardwaj V., Ohri P., Sharma A., Ahmad A., Bhardwaj R., Ahmad P. Herbal immune-boosters: Substantial warriors of pandemic COVID-19 battle. Phytomedicine. 2021;85 doi: 10.1016/j.phymed.2020.153361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Komoda M. For safer use of ivermectin in all patients. Yakugaku zasshi. 2021;141:887–909. doi: 10.1248/yakushi.20-00242. Japanese. [DOI] [PubMed] [Google Scholar]
- Li L.C., Kan L.D. Traditional Chinese medicine for pulmonary fibrosis therapy: Progress and future prospects. J. Ethnopharmacol. 2017;198:45–63. doi: 10.1016/j.jep.2016.12.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li K., An W., Xia F., Chen M., Yang P., Liao Y., Xu X., Zhou Q., Fang S., Zhang M. Observation on clinical effect of modified Qingfei Paidu Decoction in treatment of COVID-19. Chin. Tradit. Herb. Drug. 2020;51:2046–2049. doi: 10.7501/j.issn.0253-2670.2020.08.008. Chinese. [DOI] [Google Scholar]
- Li Y.D., Zhang W. Evaluation on the clinical effect of traditional Chinese medicine and western medicine regimens on COVID-19. Guangming J. Chin. Med. 2020;35:1273–1275. doi: 10.3969/j.issn.1003-8914.2020.09.001. Chinese. [DOI] [Google Scholar]
- Liu M., Gao Y., Yuan Y., Yang K., Shi S., Zhang J., Tian J. Efficacy and safety of integrated traditional Chinese and Western medicine for corona virus disease 2019 (COVID-19): a systematic review and meta-analysis. Pharmacol. Res. 2020;158 doi: 10.1016/j.phrs.2020.104896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu S.H., Ma Y., Shi N.N., Tong L., Zhang L., Chen R.B., Fan Y.P., Ji X.Y., Ge Y.W., Zhang H.M., Wang Y.P., Wang Y.Y. Qingfei Paidu Decoction for COVID-19: A bibliometric analysis. Biomed. Environ. Sci. 2021;34:755–760. doi: 10.3967/bes2021.105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Z., Du S., Shao F., Li H., Xu S., Ma X., Xu Z., Cui H., Yu C., Wu Y., Wang F., Li L., Chen R., Qiu H., Tang Z., Sun P. Efficacy of Qingfei Paidu Decoction on patients with COVID-19 pneumonia in wuhan, China: A propensity score matching study. Evid. Based Complementary Altern. Med. 2021 doi: 10.1155/2021/4303380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo H., Yang M., Tang Q.L., Hu X.Y., Willcox M.L., Liu J.P. Characteristics of registered clinical trials on traditional Chinese medicine for coronavirus disease 2019 (COVID-19): A scoping review. Eur. J. Integr. Med. 2021;41 doi: 10.1016/j.eujim.2020.101251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moher D., Liberati A.T.J., Tetzlaff J.F., Altman D.G., Altman D.G. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med. 2009;6:1–6. doi: 10.1371/journal.pmed.1000097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma Y., Zhu D.S., Chen R.B., Shi N.N., Liu S.H., Fan Y.P., Wu G.H., Yang P.Y., Bai J.F., Chen H., Chen L.Y., Feng Q., Guo T.M., Hou Y., Hu G.F., Hu X.M., Hu Y.H., Huang J., Huang Q.H., Huang S.Z., Ji L., Jin H.H., Lei X., Li C.Y., Li M.Q., Li Q.T., Li X.Y., Liu H., Liu J.P., Liu Z., Ma Y.T., Mao Y., Mo L.F., Na H., Wang J.W., Song F.L., Sun S., Wang D.T., Wang M.X., Wang X.Y., Wang Y.Z., Wang Y.D., Wu W., Wu L.P., Xiao Y.H., Xie H.J., Xu H.M., Xu S.F., Xue R.X., Yang C., Yang K.J., Yuan S.L., Zhang G.Q., Zhang J.B., Zhang L.S., Zhao S.S., Zhao W.Y., Zheng K., Zhou Y.C., Zhu J.T., Zhu T.Q., Zhang H.M., Wang Y.P., Wang Y.Y. Association of overlapped and un-overlapped comorbidities with COVID-19 severity and treatment outcomes: A retrospective cohort study from nine provinces in China. Biomed. Environ. Sci. 2020;33:893–905. doi: 10.3967/bes2020.123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma Y., Mishra S.R., Han X.K., Zhu D.S. The relationship between time to a high COVID-19 response level and timing of peak daily incidence: an analysis of governments' stringency index from 148 countries. Infect. Dis. Poverty. 2021;10:96. doi: 10.1186/s40249-021-00880-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NHC (the National Health Commission) 2020. Progress on screening of effective prescription of traditional Chinese medicine on Feb 6, 2020.http://bgs.satcm.gov.cn/gongzuodongtai/2020-02-06/12866.html Chinese. [Google Scholar]
- NHC (the National Health Commission) NHC; 2020. Notice on the issuance of the new 6th version of the COVID-19 Diagnosis and Treatment Guideline.http://www.satcm.gov.cn/d/file/p/2020/02-19/af5165ab83bc961eb8c20f883c4dd594.pdf Chinese. [Google Scholar]
- Ni L., Chen L., Huang X., Han C., Xu J., Zhang H., Luan X., Zhao Y., Xu J., Yuan W., Chen H. Combating COVID-19 with integrated traditional Chinese and Western medicine in China. Acta Pharm. Sin. B. 2020;10:1149–1162. doi: 10.1016/j.apsb.2020.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pan H., Peto R., Henao-Restrepo A.M., Preziosi M.P., Sathiyamoorthy V., Abdool Karim Q., Alejandria M.M., Hernández García C., Kieny M.P., Malekzadeh R., Murthy S., Reddy K.S., Roses Periago M., Abi Hanna P., Ader F., Al-Bader A.M., Alhasawi A., Allum E., Alotaibi A., Alvarez-Moreno C.A., Appadoo S., Asiri A., Aukrust P., Barratt-Due A., Bellani S., Branca M., Cappel-Porter H.B.C., Cerrato N., Chow T.S., Como N., Eustace J., García P.J., Godbole S., Gotuzzo E., Griskevicius L., Hamra R., Hassan M., Hassany M., Hutton D., Irmansyah I., Jancoriene L., Kirwan J., Kumar S., Lennon P., Lopardo G., Lydon P., Magrini N., Maguire T., Manevska S., Manuel O., McGinty S., Medina M.T., Mesa Rubio M.L., Miranda-Montoya M.C., Nel J., Nunes E.P., Perola M., Portolés A., Rasmin M.R., Raza A., Rees H., Reges P.P.S., Rogers C.A., Salami K., Salvadori M.I., Sinani N., Sterne J.A.C., Stevanovikj M., Tacconelli E., Tikkinen K.A.O., Trelle S., Zaid H., Røttingen J.A., Swaminathan S. Repurposed antiviral drugs for COVID-19 - Interim WHO solidarity trial results. N. Engl. J. Med. 2020;6:497–511. doi: 10.1056/NEJMoa2023184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ren J.L., Zhang A.H., Wang X.J. Traditional Chinese medicine for COVID-19 treatment. Pharmacol. Res. 2020;155 doi: 10.1016/j.phrs.2020.104743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slim K., Nini E., Forestier D., Kwiatkowski F., Panis Y., Chipponi J. Methodological index for non-randomized studies (MINORS): development and validation of a new instrument. ANZ J. Surg. 2003;73:712–716. doi: 10.1046/j.1445-2197.2003.02748.x. [DOI] [PubMed] [Google Scholar]
- SATCM (the State Administration of Traditional Chinese Medicine) 2020. Notice on the recommendation of the use of Qingfei Paidu Decoction in the treatment of COVID-19 by integrated traditional Chinese and Western medicine.http://yzs.satcm.gov.cn/zhengcewenjian/2020-02-07/12876.html Chinese. [Google Scholar]
- Shi N., Liu B., Liang N., Ma Y., Ge Y., Yi H., Wo H., Gu H., Kuang Y., Tang S., Zhao Y., Tong L., Liu S., Zhao C., Chen R., Bai W., Fan Y., Shi Z., Li L., Liu J., Gu H., Zhi Y., Wang Z., Li Y., Li H., Wang J., Jiao L., Tian Y., Xiong Y., Huo R., Zhang X., Bai J., Chen H., Chen L., Feng Q., Guo T., Hou Y., Hu G., Hu X., Hu Y., Huang J., Huang Q., Huang S., Ji L., Jin H., Lei X., Li C., Wu G., Li J., Li M., Li Q., Li X., Liu H., Liu J., Liu Z., Ma Y., Mao Y., Mo L., Na H., Wang J., Song F., Sun S., Wang D., Wang M., Wang X., Wang Y., Wang Y., Wu W., Wu L., Xiao Y., Xie H., Xu H., Xu S., Xue R., Yang C., Yang K., Yang P., Yuan S., Zhang G., Zhang J., Zhang L., Zhao S., Zhao W., Zheng K., Zhou Y., Zhu J., Zhu T., Li G., Wang W., Zhang H., Wang Y., Wang Y. Association between early treatment with Qingfei Paidu Decoction and favorable clinical outcomes in patients with COVID-19: A retrospective multicenter cohort study. Pharmacol. Res. 2020;161:1–10. doi: 10.1016/j.phrs.2020.105290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun Y., Lv W., Li H., Xiao Y., Yang M., Yang H., Gao Q., Yang Z., Shou Z., Hu J., Ma Y., Luo Z., Chen B., Liu L., Shen F., Zhang S., Liu Z., Xu X., Zhao Z., Zhang H., Long Y., Mei Q., Shi R., Liu H. Multicenter clinical research of Qingfei Paidu Decoction in 295 cases in the treatment of COVID-19. J. Tradit. Chin. Med. 2021;7:599–603. doi: 10.13288/j.11-2166/r.2021.07.010. Chinese. [DOI] [Google Scholar]
- Tian J., Yan S., Wang H., Zhang Y., Zheng Y., Wu H., Li X., Gao Z., Ai Y., Gou X., Zhang L., He L., Lian F., Liu B., Tong X. Hanshiyi Formula, a medicine for Sars-CoV-2 infection in China, reduced the proportion of mild and moderate COVID-19 patients turning to severe status: A cohort study. Pharmacol. Res. 2020;161 doi: 10.1016/j.phrs.2020.105127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang R.Q., Yang S.J., Xie C.G., Shen Q.L., Li M.Q., Lei X., Li J.K., Huang M. Clinical observation on Qingfei Paidu Decoction in treating COVID-19. Chin. Med. Pharm. Clin. 2020;36:13–18. doi: 10.13412/j.cnki.zyyl.20200303.002. Chinese. [DOI] [Google Scholar]
- Wang Y., Chen L., Zhang L., Ku B.Q., Yu R., Zhang X.F. Clinical effects of Qingfei Paidu Decoction combined with conventional treatment on patients with coronavirus disease 2019. Chin. Tradit. Pat. Med. 2021;43:656–659. doi: 10.3969/j.issn.1001-1528.2021.03.017. Chinese. [DOI] [Google Scholar]
- Wang Q., Zhu H., Li M., Liu Y., Lai H., Yang Q., Cao X., Ge L. Efficacy and safety of Qingfei Paidu Decoction for treating COVID-19: A systematic review and meta-analysis. Front. Pharm. 2021;12 doi: 10.3389/fphar.2021.688857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xin S., Cheng X., Zhu B., Liao X., Yang F., Song L., Shi Y., Guan X., Su R., Wang J., Xing L., Xu X., Jin L., Liu Y., Zhou W., Zhang D., Liang L., Yu Y., Yu R. Clinical retrospective study on the efficacy of Qingfei Paidu Decoction combined with Western medicine for COVID-19 treatment. Biomed. Pharmacother. 2020;129 doi: 10.1016/j.biopha.2020.110500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiong X., Wang P., Su K., Cho W.C., Xing Y. Chinese herbal medicine for coronavirus disease 2019: A systematic review and meta-analysis. Pharmacol. Res. 2020;160 doi: 10.1016/j.phrs.2020.105056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu D., Xu Y., Wang Z., Lv Y., Zhu H., Song T. Mechanism of Qingfei Paidu Decoction on COVID-19 based on network pharmacology. Pharmacol. Clin. Tradit. Chin. Med. 2020;36:26–32. doi: 10.13412/j.cnki.zyyl.20200305.001. Chinese. [DOI] [Google Scholar]
- Yu H., Ren X., Qi X., Zuo Q., Liu D. Efficacy study of arbidol, Qingfei Paidu Decoction, Lianhuaqingwen Capsule, and Jinye Baidu Granules in the treatment of mild/moderate COVID-19 in a fangcang shelter hospital. Pharmacol. Clin. Chin. Materia. Med. 2020;36:2–6. doi: 10.13412/j.cnki.zyyl.20201110.005. Chinese. [DOI] [Google Scholar]
- Yu X., Zhang S., Yan F., Su D. Comparison of clinical efficacy of Qingfei Paidu Decoction combined with Western medicine in 43 cases and single Western medicine in 46 cases in the treatment of COVID-19. J. Shandong Univ. Health Sci. 2020;58:47–53. doi: 10.6040/j.issn.1671-7554.0.2020.0870. Chinese. [DOI] [Google Scholar]
- Yue D.H. Analysis of TCM epidemic diseases pathogenic theory. J. Tradit. Chin. Med. 2012;27:3044–3047. Chinese. [Google Scholar]
- Zeng X., Zhuang L., Yang Z., Dong S. Meta analysis series 7: quality assessment tools for nonrandomized experimental studies, diagnostic trials, and animal trials. Chin. J. Evid. Based Cardiovasc. Med. 2012;4:496–499. doi: 10.3969/j.1674-4055.2012.06.003. Chinese. [DOI] [Google Scholar]
- Zeng X., Ma W., Wang J. Effect of Qingfei Paidu Decoction on clinical efficacy of COVID-19 pneumonia with phlegm heat blocking lung. Med. J. West China. 2020;32 doi: 10.3969/j.issn.1672-3511.2020.12.017. 1799–1801+1806. Chinese. [DOI] [Google Scholar]
- Zhao J., Tian S., Lu D., Yang J., Zeng H., Zhang F., Tu D., Ge G., Zheng Y., Shi T., Xu X., Zhao S., Yang Y., Zhang W. Systems pharmacological study illustrates the immune regulation, anti-infection, anti-inflammation, and multi-organ protection mechanism of Qingfei Paidu Decoction in the treatment of COVID-19. Phytomedicine. 2020;85 doi: 10.1016/j.phymed.2020.153315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhong L.L.D., Lam W.C., Yang W., Chan K.W., Sze S.C.W., Miao J., Yung K.K.L., Bian Z., Wong V.T. Potential targets for treatment of coronavirus disease 2019 (COVID-19): A review of Qingfei Paidu Decoction and its major herbs. Am. J. Chin. Med. 2020;48:1051–1071. doi: 10.1142/S0192415X20500512. [DOI] [PubMed] [Google Scholar]
- Zhang L., Zheng X., Bai X., Wang Q., Chen B., Wang H., Lu J., Hu S., Zhang X., Zhang H., Liu J., Shi Y., Zhou Z., Gan L., Li X., Li J. Association between use of Qingfei Paidu Decoction and mortality in hospitalized patients with COVID-19: A national retrospective registry study. Phytomedicine. 2021;85 doi: 10.1016/j.phymed.2021.153531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang P., Pan G.T. Clinical study on the improvement of inflammatory cytokines in critically ill patients with COVID-19 treated by Qingfei Paidu Decoction. Mod. Tradit. Chin. Med.-WST. 2021;23:391–395. doi: 10.11842/wst.20200416003. Chinese. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.