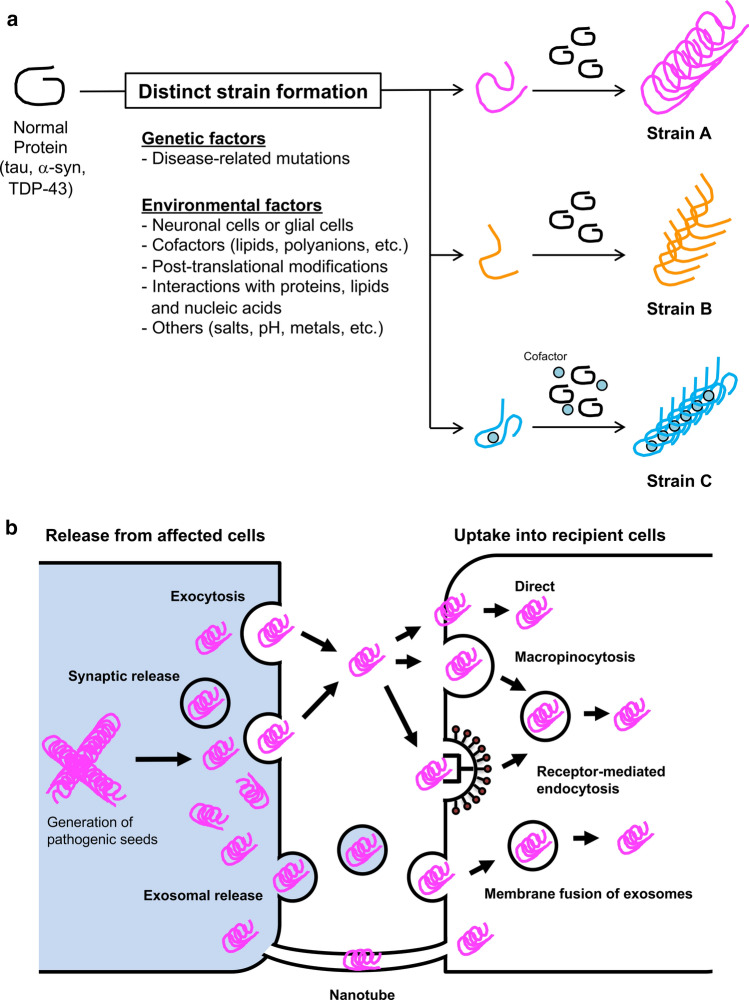
Fig. 5.
Strain formation and cell-to-cell transmission in neurodegenerative diseases. a Prion-like proteins, including tau, α-syn and TDP-43, can adopt various misfolded forms. The variety of misfolding is caused by genetic and/or environmental factors, resulting in the formation of strains with distinct conformations. Disease-associated mutations alter the core structure of filaments and the interaction between two protofilaments. Differences in the cellular environment between neuronal and glial cells may also contribute to the various types of misfolding. The interaction of prion-like proteins with cell type-specific co-factors during misfolding would lead to the formation of disease-specific filaments. Post-translational modifications, interactions with other proteins, lipids and nucleic acids, as well as differences in salt concentration, pH and metals, may also be involved in the formation of distinct strains. b Pathogenic proteins amplified and accumulated in cells have proposed to transmit as seeds from cell to cell and then spread throughout the brain. Possible mechanisms of cell-to-cell transmission include extracellular release of pathogenic seeds via exocytosis or in synaptic vesicles or exosomes, followed by incorporation into neighboring cells either directly or via macropinocytosis or receptor-mediated endocytosis. Alternatively pathogenic seeds may be taken up into cells by cell membrane fusion of exosomes containing seeds. Cell-to-cell transmission of pathogenic seeds via tunneling nanotubes has also been suggested