Figure 3. Most hinge variants have comparable in vitro cytolytic capacity and expansion relative to the native CAR.
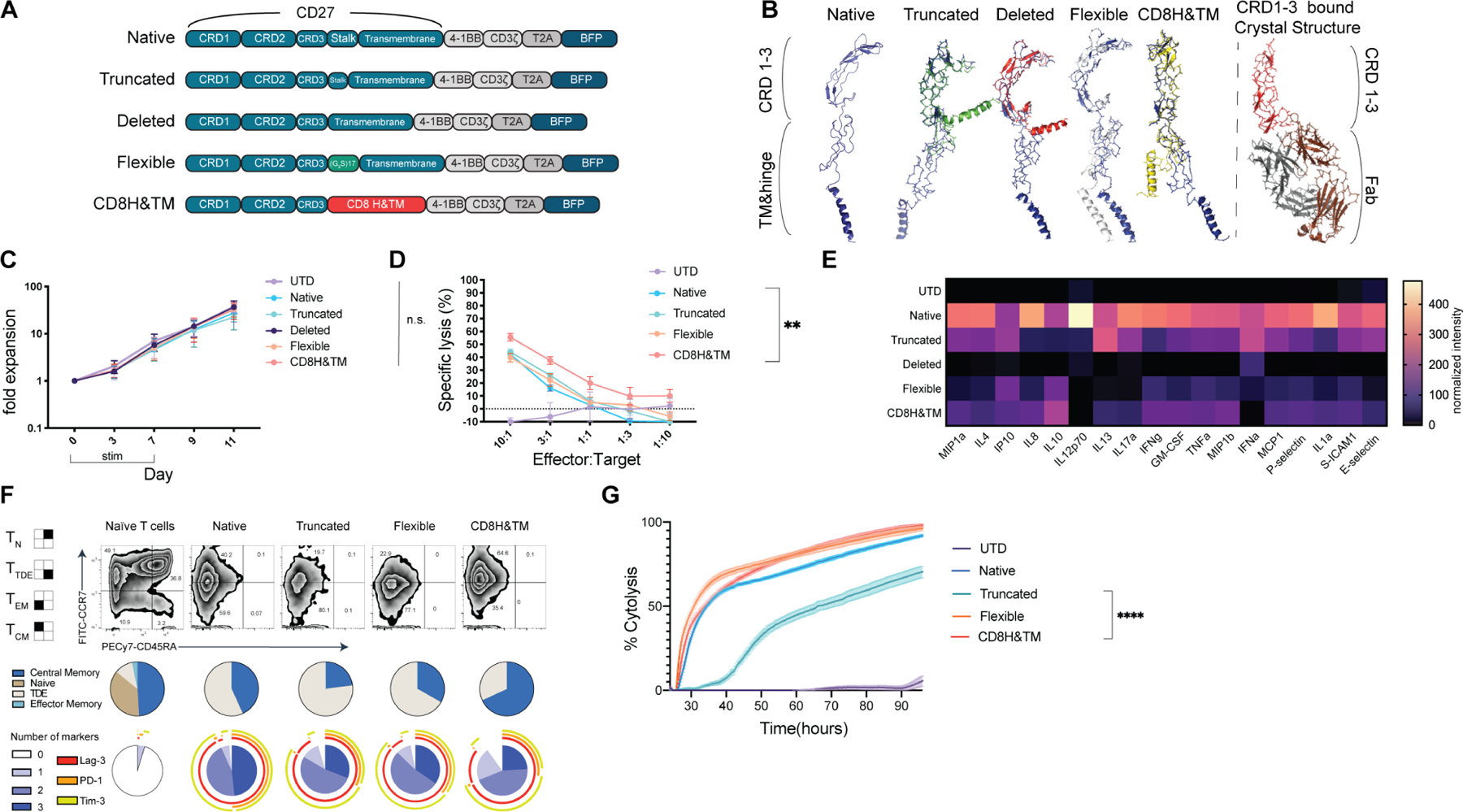
(A) Construct designs for CD70-targted CAR hinge variants. (B) Predicted structure of the CD70-targeted CAR cysteine rich domains (CRDs) as well as the hinge and transmembrane domains using the Phyre2 (Kelley et al., 2015) predictive engine with each aligned in Pymol3 with the MUSCLE plugin to the Native CRD (blue structure). Published crystal structure for CRD 1,2, & 3 is shown for comparison binding to a Fab. (C) CD70 hinge variant CAR expansion after lentiviral transduction. All differences are nonsignificant (ns) by 2-way ANOVA with Dunnett’s correction for multiple hypotheses. Points represent mean ± SEM of T-cells from 3 healthy donors. (D) Cytotoxicity as assessed in a luciferase-based killing assay for 16hrs with the indicated CAR and OCI-AML3 target. Data points indicate mean ± SEM of technical triplicates also performed in biological triplicate with T-cells derived from 3 healthy donors. * p< 0.05, **** p< 0.0001 by one-way ANOVA with Dunnett’s multiple hypotheses correction at the 10:1 E:T ratio compared to the native CAR. (E) Heatmap of normalized cytokines in the supernatants of CD70-CAR hinge variants after overnight co-culture with Molm13 at a 1:1 ratio. Cytokines were measured by Luminex assay in technical duplicates and biological triplicate with T cells derived from 3 healthy donors. Representative of n=1 independent experiment. (F) CD70-CAR hinge variants were stimulated with 5 doses of weekly CD70-expressing irradiated K562s at a 1:1 ratio and assessed at day 28 for phenotype and exhaustion markers. T cells from the corresponding unstimulated healthy donor are shown for comparison. Exhaustion markers from naïve T cells from the same unstimulated healthy donor are shown for reference. Performed in 3 independent donors, data is representative of a single donor. (G) The same CARs from day 28 were used in a real-time cytotoxicity assay against Molm13 targets at a 1:1 effector: target ratio (relative to Day 0 tumor seeding). Assay performed in quadruplicate. Means are plotted ± SEM. **** p <0.0001 by one-way ANOVA assessed at the terminal time point.
See also Figure S4.
