Figure 1. Design of synthetic RIP receptors for customized antigen-dependent gene regulation in therapeutic cells.
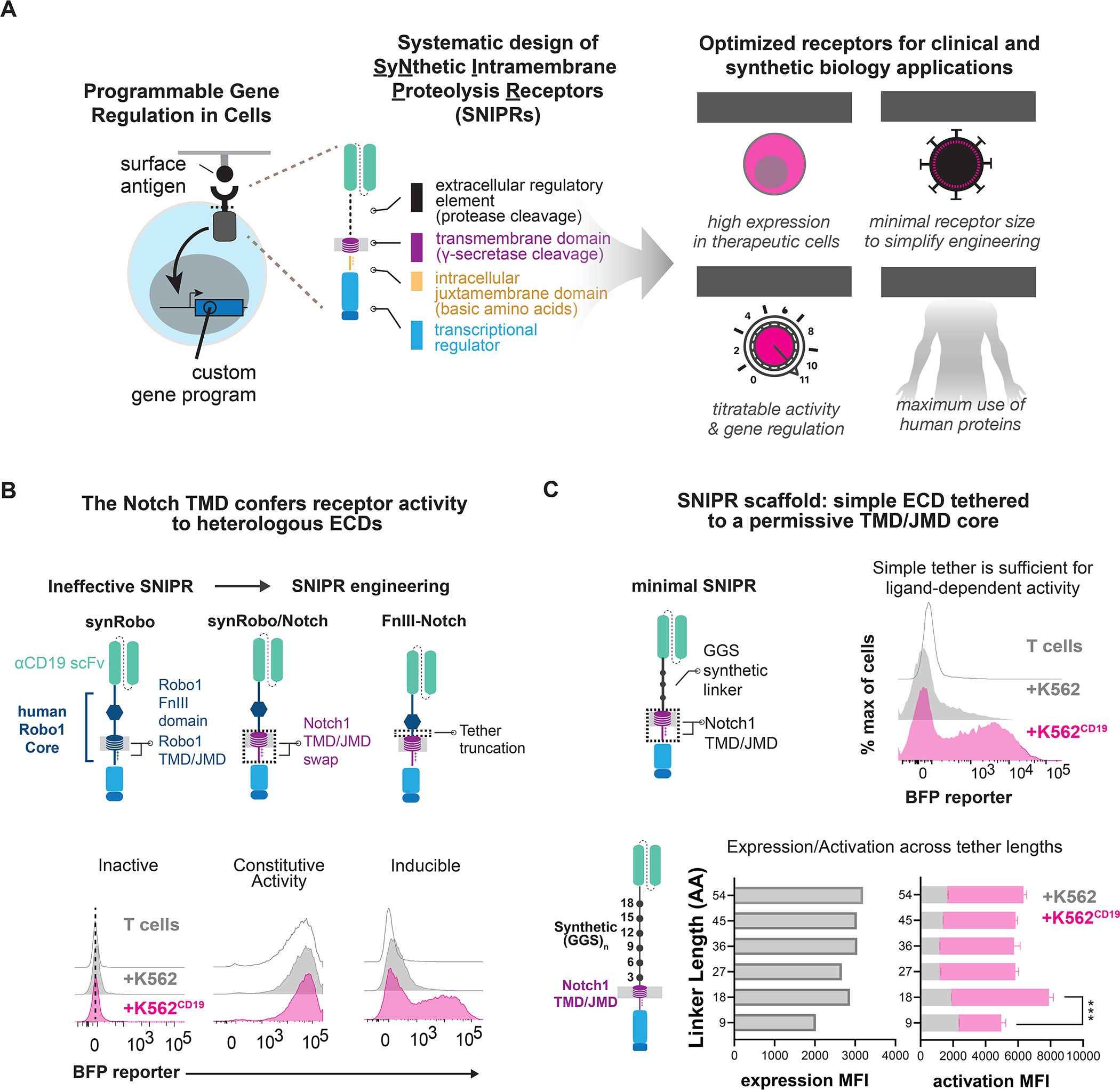
(A) Design of Synthetic Intramembrane Proteolysis Receptors. Receptors are comprised of a ligand binding domain (LBD), an extracellular domain (ECD), a transmembrane domain (TMD), a juxtamembrane domain (JMD), and a transcription factor (TF). Receptor circuits are designed to maximize clinical translation potential (B). A synRobo receptor replaces the Notch1 core with one from human Robo1. Compared to synNotch, a synRobo receptor fails to induce BFP. By replacing the TMD and JMD of Robo1 with those of Notch1, control of BFP production is lost. Deletion of a known ADAM10 cleavage site in the Robo1 ECD rescues ligand-dependent receptor behavior. (C) Same as B, but with minimal SNIPRs constructed using simple (GGS)n ECDs, and the TMD/JMD from Notch1. Statistics were calculated using unpaired T-tests, ***P≤0.001.
