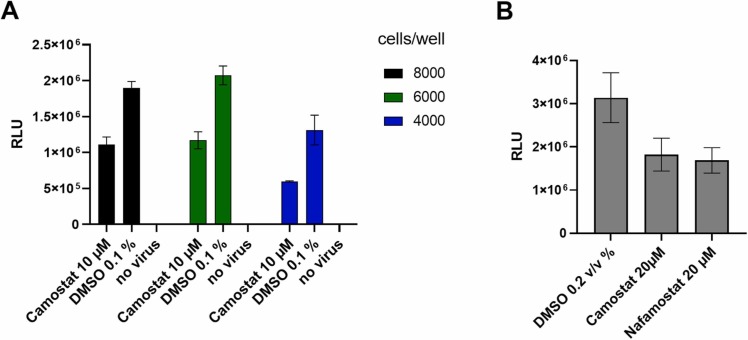
Fig. 1.
: Adaptation and pharmacological validation of SARS-CoV-2 pseudovirus assay for screening, (A). Titration of cell number per well in a 384-well plate, calculation of signal/background ratio using camostat as positive control and DMSO as negative control (B). Validation of assay response to known inhibitors of SARS-CoV-2 entry. Compounds were added 30 min prior to virus addition to 8000 cells per well. Data shown is based on the average and standard deviation of three independent experiments. Detection of luminescence signal was performed after 48 h at 37 °C. RLU: relative light units measured as counts per 100 ms/well.