Figure 1.
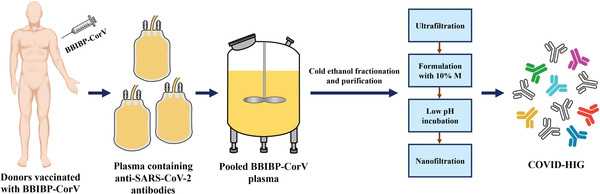
Workflow for COVID‐HIG production. Plasma containing anti‐SARS‐CoV‐2 antibodies was collected from immunized healthy donors who were vaccinated with BBIBP‐CorV (two‐dose). The plasma samples were combined to obtain pooled BBIBP‐CorV plasma. Then, pooled BBIBP‐CorV plasma was fractionated and purified to prepare COVID‐HIG by commercial cold ethanol fractionation. M, maltose. COVID‐HIG, COVID‐19 hyperimmune globulin.
