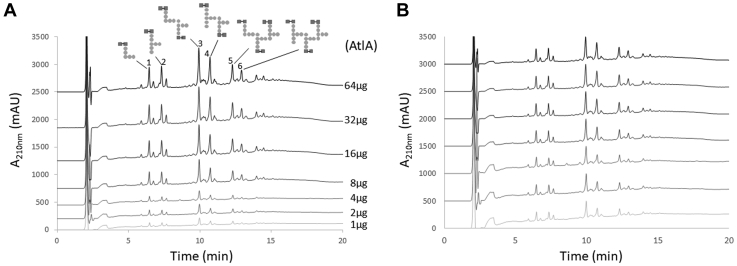
Figure 3.
Reversed-phase HPLC analysis of muropeptides solubilized by AtlA.A, Enterococcus faecalis peptidoglycan was digested by increasing amounts of recombinant AtlA (from 1 to 64 μg). Soluble fragments released were reduced and separated on a Hypersil C18 column, showing a mixture of monomers and multimers. The schematic structure of major monomers (peaks 1 and 2), dimers (peaks 3 and 4), and trimers (peaks 5 and 6) is shown. GlcNAc and MurNAc are shown as filled squares, and peptide stem residues are shown as filled circles. B, all traces were normalized to display the same amplitude for the major dimer, showing no preferential release of monomeric structures and an overall very similar profile, irrespective of the amount of enzyme used.