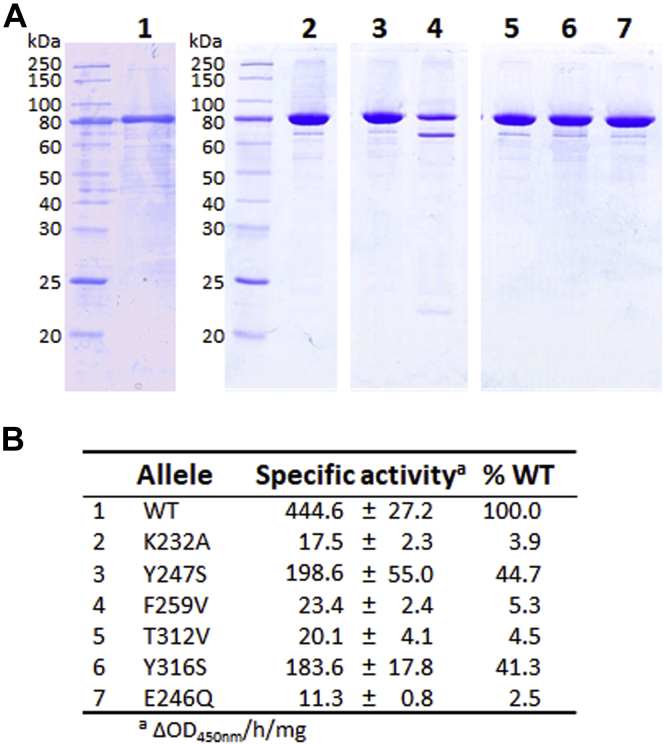
Figure 5.
Purification and characterization of recombinant AtlA enzymatic activity.A, SDS-PAGE analysis of full-length recombinant AtlA proteins. AtlA variants were produced in Escherichia coli and purified using a one-step immobilized metal affinity chromatography; lane 1, WT; lane 2, K232A; lane 3, Y247S; lane 4, F259V; lane 5, T312V; lane 6, Y316S; and lane 7, E246Q. B, specific activity (expressed as Δabsorbance at 450 nm/h/mg) was measured using Micrococcus luteus as a substrate.