Scheme 2. Synthesis of tert-Butyl 4-((5-Aminopyrimidin-2-yl)methyl)piperazine-1-carboxylate 17.
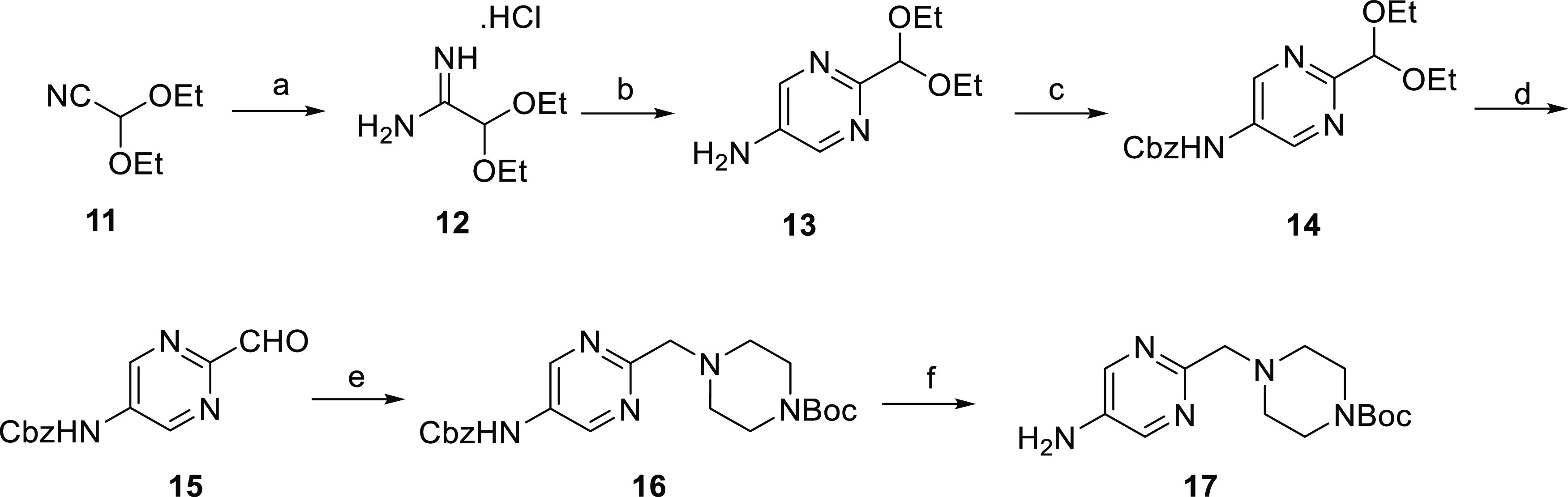
Reagents and conditions: (a) (i) NaOMe, MeOH, RT, 16 h; (ii) NH4Cl, MeOH, RT, 18 h, 89%; (b) (i) N-(3-(dimethylamino)-2-[[(dimethylamino)methylene]amino]prop-2-en-1-ylidene)-N-methylmethanaminium hydrogen dihexafluorophosphate, NaOMe (1 M in MeOH), EtOH, 78 °C, 2.5 h; (ii) 5% aq K2CO3, dioxane, 100 °C, 18 h, 49% (over two steps); (c) benzyl chloroformate, K2CO3, THF/H2O (1:1), RT, 24 h, 80%; (d) HCl (1 M aq), MeCN, RT, 8 h, 89%; (e) (i) tert-butyl piperazine-1-carboxylate, MgSO4, 2,2,2-trifluoroethanol, 1 h, RT; (ii) NaBH4, 2,2,2-trifluoroethanol, 0 °C to RT, 1 h, 42%; and (f) H2, 10% Pd/C, EtOAc, RT, 24 h, 99%.
