Scheme 3. Synthesis of tert-Butyl 4-((5-Aminopyridin-2-yl)methyl)piperazine-1-carboxylate 24.
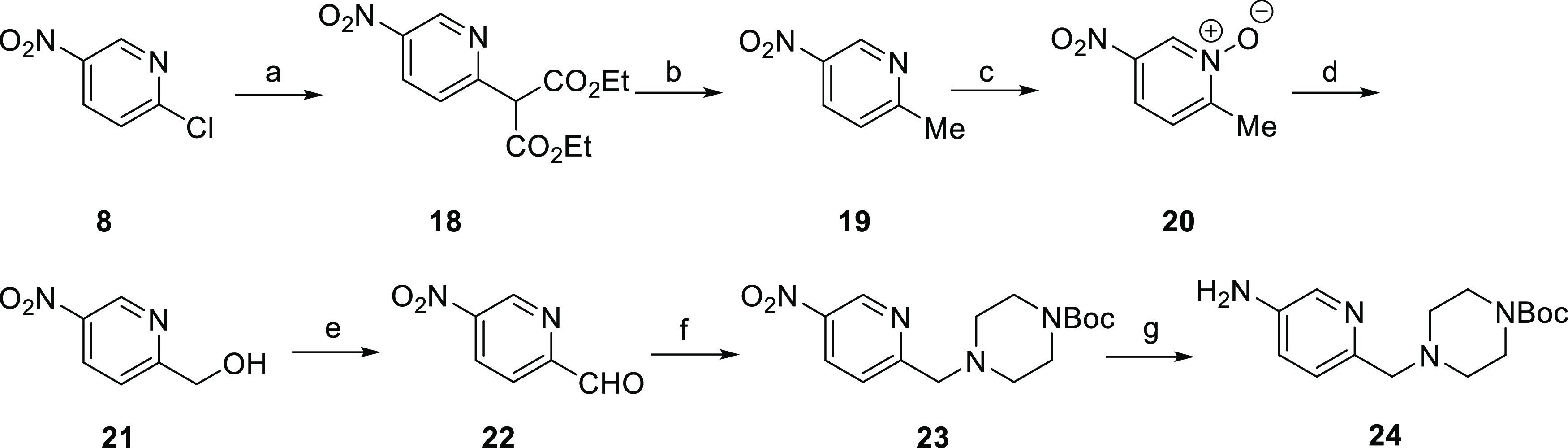
Reagents and conditions: (a) (i) NaH (60% dispersion in mineral oil), diethyl malonate, THF, 0 °C to RT, 1 h; (ii) 2-chloro-5-nitropyridine, 0 °C to RT, 20 h, 64%; (b) 20% aq H2SO4, 100 °C, 2 h, 95%; (c) m-CPBA (74%), dichloromethane (DCM), 0 °C to RT, 16 h, 96%; (d) (i) trifluoroacetic anhydride (TFAA), DCM, 0 °C to RT, 16 h; (ii) MeOH, 0 °C to RT, 8 h, 50%; (e) MnO2, DCM, RT, 16 h, 61%; (f) (i) tert-butyl piperazine-1-carboxylate, MgSO4, 2,2,2-trifluoroethanol, 1 h, 38 °C; (ii) NaBH4, 2,2,2-trifluoroethanol, 0 °C to RT, 1 h, 51%; and (g) H2, 10% Pd/C, MeOH/THF (1:1), 40 °C, 8 h, 95%.
