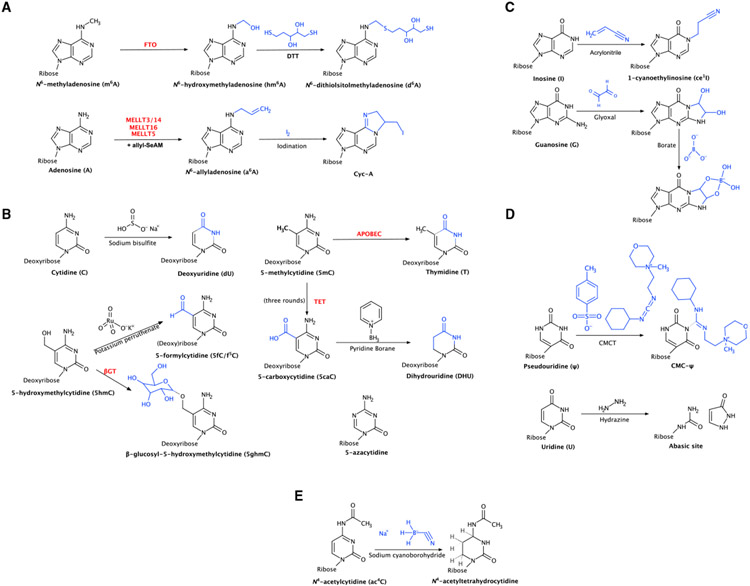
Figure 2. Chemical and biochemical reactions for each technique discussed.
(A) (Bio)chemical modifications used in the study of m6A: biochemical modification of m6A to hm6A by the enzyme FTO and subsequent chemical modification to d6A by DTT and biochemical modification of A to a6A by writer enzymes and subsequent chemical modification to Cyc-A by iodination.
(B) (Bio)chemical modifications used in the study of 5mC/m5C: chemical deamination of C to dU by bisulfite, chemical oxidation of 5hmC to 5fC by KRuO4 and biochemical modification of 5hmC to 5ghmc by the enzyme β-GT, biochemical deamination of 5mC to T by APOBEC, chemical modification of 5caC to dihydrouridine (DHU) by pyridine borane, and structure of 5-azacytidine.
(C) Chemical modifications used in the study of inosine: chemical modification of inosine to ce1I by acrylonitrile and chemical modification of guanosine with glyoxal and further modification with borate.
(D) Chemical modifications used in the study of ψ: chemical modification of ψ with CMCT and chemical cleavage of uridine with hydrazine.
(E) Chemical modification used in the study of ac4C: chemical reduction of ac4C by NaCNBH3.