Abstract
Placenta forms as a momentary organ inside the uterus with a slew of activities only when the woman is pregnant. It is a discoid-shaped hybrid structure consisting of maternal and embryonic components. It develops in the mesometrial side of the uterus following blastocyst implantation to keep the two genetically different entities, the mother and embryo, separated but connected. The beginning and progression of placental formation and development following blastocyst implantation coincides with the chronological developmental stages of the embryo. It gradually acquires the ability to perform the vascular, respiratory, hepatic, renal, endocrine, gastrointestinal, immune, and physical barrier functions synchronously that are vital for fetal development, growth, and safety inside the maternal environment. The uterus ejects the placenta when its embryonic growth and survival supportive roles are finished; that is usually the birth of the baby. Despite its irreplaceable role in fetal development and survival over the post-implantation progression of pregnancy, it still remains unclear how it forms, matures, performs all of its activities, and starts to fail functioning. Thus, a detailed understanding about normal developmental, structural, and functional aspects of the placenta may lead to avoid pregnancy problems that arise with the placenta.
Keywords: Blastocyst, Decidua, Implantation, Labyrinth, Placenta
1. Introduction
Placenta is a vital organ for the development and growth of the fetus from the time of implantation to birth. It is responsible for fetal respiratory, nutritional, excretory, endocrine, and immunological functions. It also has an extraordinary ability to adapt to adverse maternal and environmental cues and diminishes their impact on the fetus. It starts to form and grow at the interface of the uterine endometrium and blastocyst following a successful institution of the blastocyst–uterine contact, known as the implantation process. Placenta development, growth and maturation are directedly linked to the blastocyst’s morphogenetic developmental advances to the stages of first the egg cylinder, then the gastrula, and lastly the fetus (Rossant and Cross 2001).
Placental developmental, positional, and functional defects are threats to pregnancy complications such as early pregnancy failure, stillbirth, fetal growth restriction, preeclampsia, and preterm birth. Some pregnancy complications that arise late in pregnancy such as preeclampsia and preterm birth may reflect errors that occur during early in placental development (Burton et al. 2016; Hemberger et al. 2020). There are several major placental abnormalities based on the site of implantation, degree of trophoblast invasion, and functions of the placenta. The placenta normally forms onto the upper part of the uterus in humans. However, when it attaches too deeply it causes problems such as the placenta accreta (firm attachment with the myometrium), increta (penetration inside the myometrium), and percreta (breach through the myometrium to nearby organs) (Bauer and Bonanno 2009; Oyelese and Smulian 2006). On the contrary, inadequate trophoblast invasion has been implicated in the pathophysiology of preeclampsia (Phipps et al. 2019). Placental previa is a condition when the placenta develops in the lower part of the uterus and partially or completely covers the cervix (Kollmann et al. 2016). Placental insufficiency is a disorder marked by insufficient blood flow to the placenta (Brown and Hay 2016; Browne 1963). Placental abruption occurs when the placenta separates from the uterus prior to the birth of the baby (Oyelese and Ananth 2006). Placental insufficiency, placental abruption, and preeclampsia may share a common pathologic mechanism involving poor placentation and placental ischemia.
Pregnancy problems due to placental defects are difficult to fix, but the fetal growth and birth complications can be managed if it is early diagnosed. By far, the placenta is an understudied organ and, hence, a least understood structure. A better understanding of the molecular mechanisms responsible for placenta formation, maturation, and functions will have a great impact on the development of treatment tools to mitigate pregnancy complications due to placental flaws. Researchers are relentlessly seeking to reveal what a normal placenta is and how it forms and performs its various roles. A brief review of previous as well as more recent works in revealing the origins of the placental cell lineages and the chronology of placental developmental events during the post-implantation period in mice is described in this article.
2. The Gist of Preimplantation Embryonic Developmental Stages
The emergence of placental cell types initiates at the preimplantation embryonic stage when the totipotent blastomeres start segregating to form trophectoderm (Tr) and inner cell mass (ICM) lineages (Guo et al. 2020). A haploid female gamete or ovum when becomes diploid (zygote) as a result of a union with a haploid male gamete (sperm) through a process called fertilization achieves the ability to undergo cleavage division and differentiation to create a live organism (Ikawa et al. 2010). Fertilization of an ovum occurs at the fimbriated end of the oviduct in mice. Mouse embryos take about 3 days to develop from the zygotic stage to the blastocyst stage (Fig. 1). The zygote begins 4 days of travel down the oviduct to the uterus. After three rounds of mitotic division inside the oviduct, the zygote progresses through the 2-cell stage and 4-cell stage to the 8-cell stage. Individual cell in each stage of these embryos is referred to as blastomere. Blastomeres are considered totipotent (ability to generate all cell-types required to form an organism) until the early 8-cell stage. Totipotent to pluripotent transition conceivably begins at this stage when the 8-cell stage embryo undergoes a process called compaction during which blastomere to blastomere contact is maximized by polarization and tight adhesion. Thus, compaction marks the beginning of first event of morphogenic and blastomere’s differentiation into two cell lineages that are found in the blastocyst. Subsequent two mitotic divisions give rise to 16-cell and 32-cell stage embryos, called the morula and blastocyst, respectively. As the 8-cell embryo transitions to 16-cell, most of the blastomeres at this stage are positioned outside encircling a few interior blastomeres. This embryonic stage is termed as the morula. The development of the morula-stage embryo in mice takes place inside the oviduct. The development of the blastocyst stage from the morula stage occurs either inside the oviduct or the uterus. There is evidence that mitotic cleavage stages of blastocyst formation vary among species. The pig and hamster embryos initiate blastocyst formation at the fourth cleavage stage (16-cell stage), a cleavage stage ahead of other species such as mice, rats, sheep, bovine, humans, and marsupials where blastocyst formation begins at or over the fifth cleavage stage (≥32-cell stage). As the morula transitions to the blastocyst stage, a fluid-filled cavity known as blastocoel (BC) appears inside the embryo when Tr cells begin to pump fluid into intracellular spaces, and further differentiation of blastomeres begins. While a small population of blastomeres that remain as a cluster inside the blastocyst is named the ICM, a layer of blastomeres that surrounds the BC and the ICM is called the Tr (Fig. 1) (Chazaud and Yamanaka 2016; Posfai et al. 2019; Rossant and Tam 2009). The number of Tr cells in an early blastocyst is always higher than ICM cells.
Fig. 1.
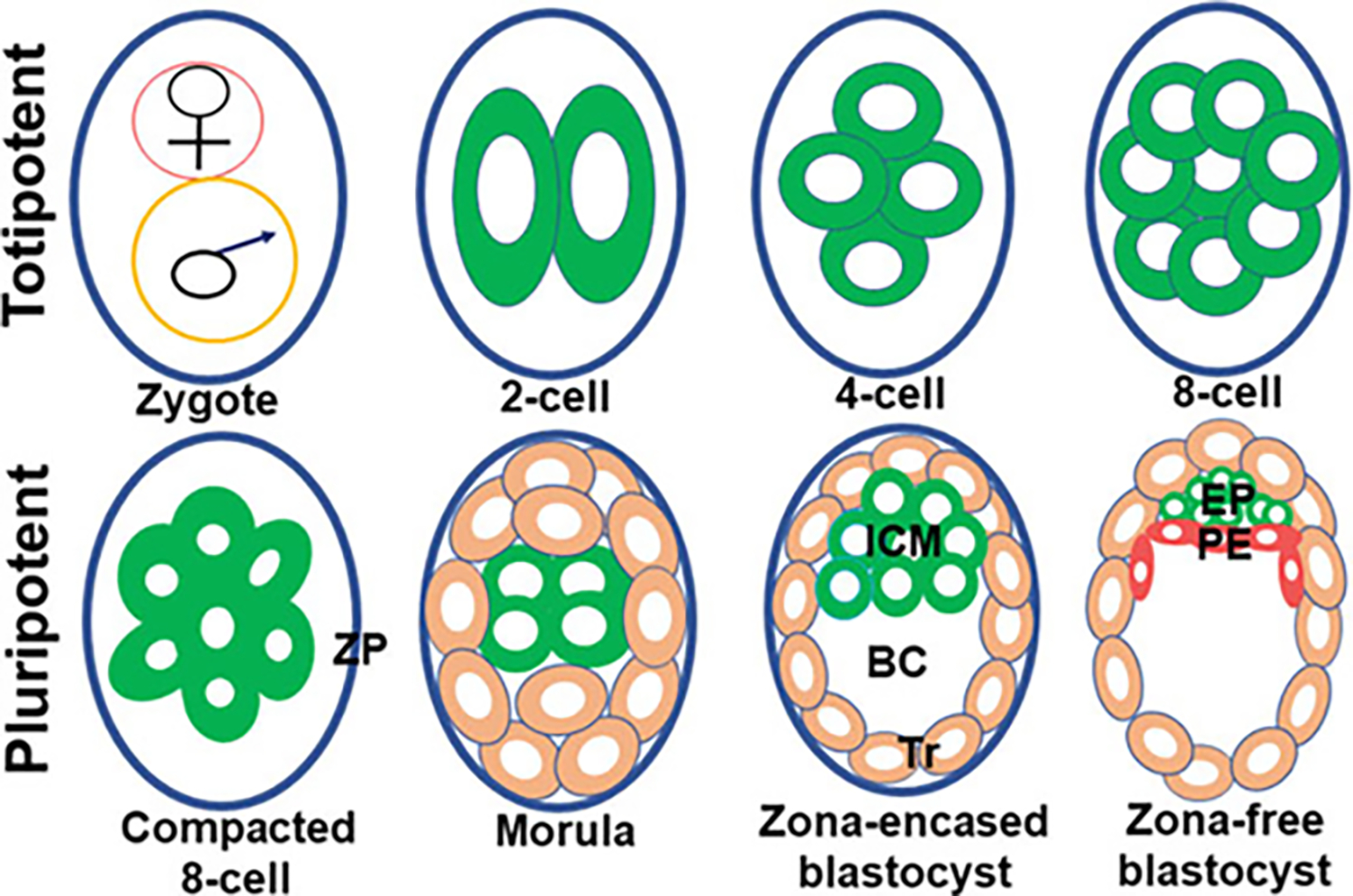
Preimplantation stages of the mouse embryo. Blastocoel (BC), Epiblast (EP), Inner cell mass (ICM), Zona pellucida (ZP), Primitive endoderm (PE), Trophectoderm (Tr)
All stages of the preimplantation embryos (1-cell stage to the blastocyst stage) remain covered by a glycoprotein envelope called the zona pellucida (ZP) (Fig. 1) and remain buoyant inside the lumens of the oviduct and uterus. The energy required for zygotic development to the 2-cell stage is derived from the nutrient stored inside the ovum. In order to continue to develop past the 2-cell stage, the embryo activates its own genome to produce energy from the prestored nutrients or nutrients sequestered from the luminal fluid of the oviduct and uterus. Recent studies suggest that embryonic gene activation may begin at the mid 1-cell embryo and dramatically increases during the 2-cell stage (Abe et al. 2018). As the blastocyst matures inside the uterine lumen, it hatches from the ZP and achieves the attachment capacity to the uterine endometrium (Yoshinaga 2013). The ICM of the blastocyst contains precursor cells for the epiblast (EP) and primitive endoderm (PE) or hypoblast. Just prior to implantation, the ICM differentiates to the EP and PE. The EP are precursors of all the cells of the embryo and extraembryonic mesoderm (Fig. 2). The PE lines the blastocoel surface of the epiblast and later generates extraembryonic endoderms (EEEn) such as the parietal and visceral endoderms (PaE and ViE) (Fig. 2). The location of the ICM or EP within the vesicular blastocyst determines the embryonic pole of the blastocyst. The domain of the blastocyst opposite to the embryonic pole is known as the abembryonic pole. Trophoblast cells that reside over the ICM and later EP are known as polar trophoblasts (Polar Tr) and the rest of trophoblast cells that surround the blastocoel are termed mural trophoblasts (Mural Tr). While polar Tr cells of the blastocyst give rise to extraembryonic ectoderm (EEE) and ectoplacental cone (EEC) following implantation, mural Tr cells generate trophoblast giant cells (TGCs) (Fig. 2) (Rossant and Cross 2001). The further development of the zona-escaped blastocyst to the fetal stage only happens when the blastocyst makes direct contact with the uterus through a process called implantation. Defects in any developmental processes of preimplantation embryos due to abnormal cell division, cell death, cell fate, and differentiation are likely causes of preimplantation embryonic death, poor quality of blastocysts, complete implantation failure, or abnormal implantation leading to placentation defects, miscarriage, preterm birth, and retarded growth of the fetus.
Fig. 2.
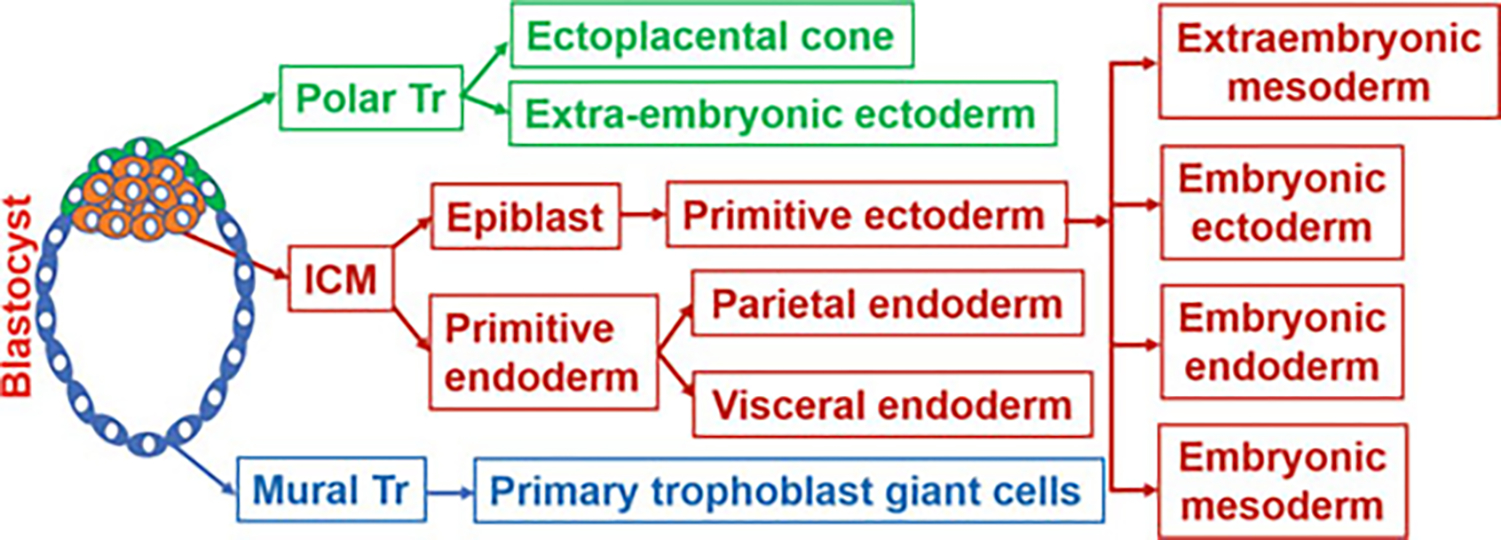
The emergence of cell lineages from the blastocyst. Inner cell mass (ICM) and trophectoderm (Tr)
3. A Gist of the Blastocyst Implantation Process
Blastocyst implantation is a landmark event of pregnancy in which a floating blastocyst makes attachment with the uterine endometrium (Figs. 3 and 4) to remain united with it until the birth. Through this process of implantation, a semiallogenic blastocyst rewards with a shelter, nutrients, oxygen, and immunological protection for its further development to the egg cylinder stage and beyond. In exchange for these services, the uterus achieves its one and only reproductive function of carrying a pregnancy in the preservation of life.
Fig. 3.
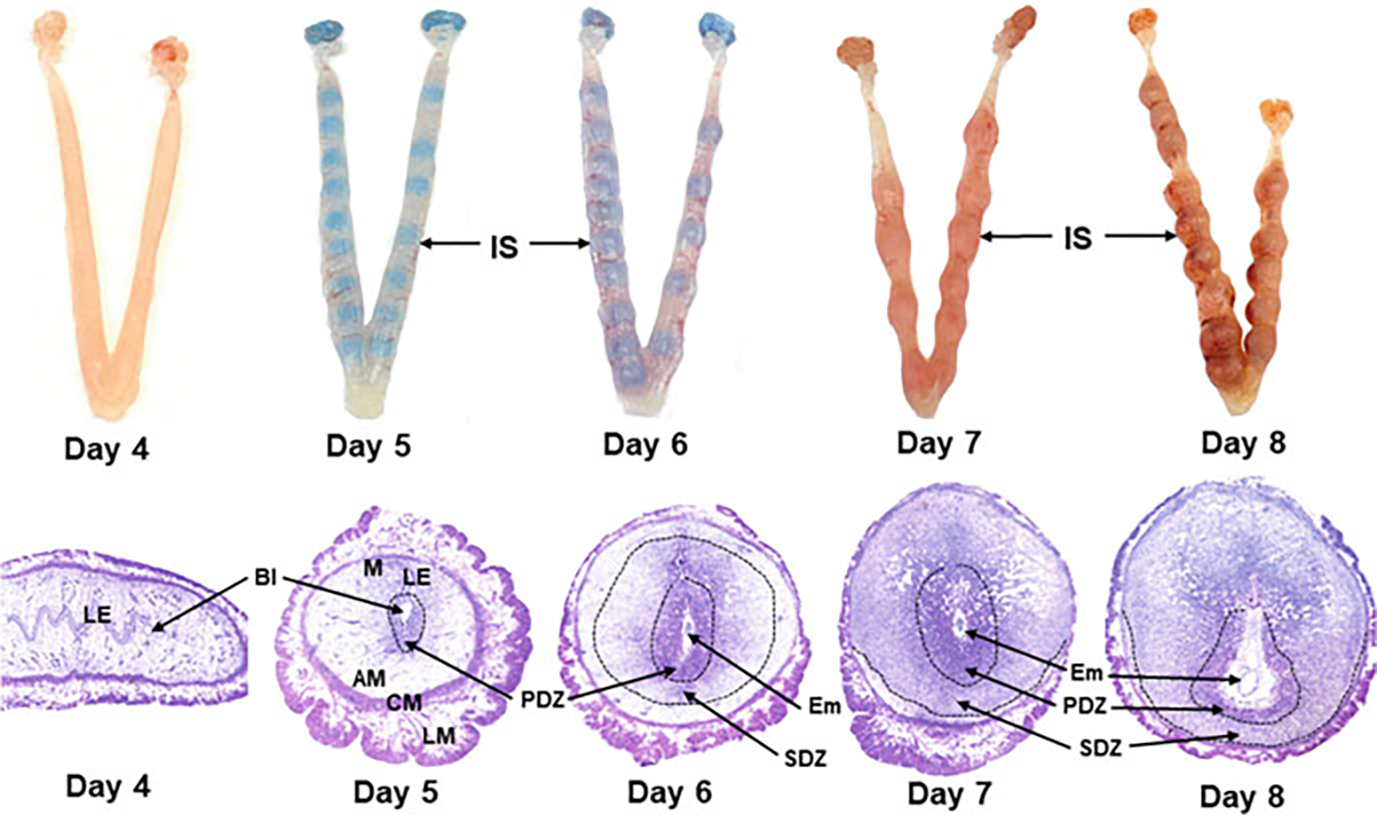
Upper panel: Photographs of the preimplantation day 4 receptive uterus and post-implantation uteri with implantation sites from days 5 to 8 of pregnancy. Blastocyst implantation sites (IS) on days 5 and 6 were visualized by intravenous injection of Chicago Blue dye. Lower panel: Photomicrographs (20×) of Hematoxylin- and Eosin-stained sections of the day 4 preimplantation uterus (longitudinal section) and implantation sites (cross sections) of days 5–8 of pregnancy. Antimesometrial (AM) side, Blastocyst (Bl), Circular muscle (CM), Embryo (Em), Longitudinal muscle (LM), Luminal epithelium (LE), Mesometrial side (M); Primary decidual zone (PDZ), Secondary decidual zone (SDZ)
Fig. 4.
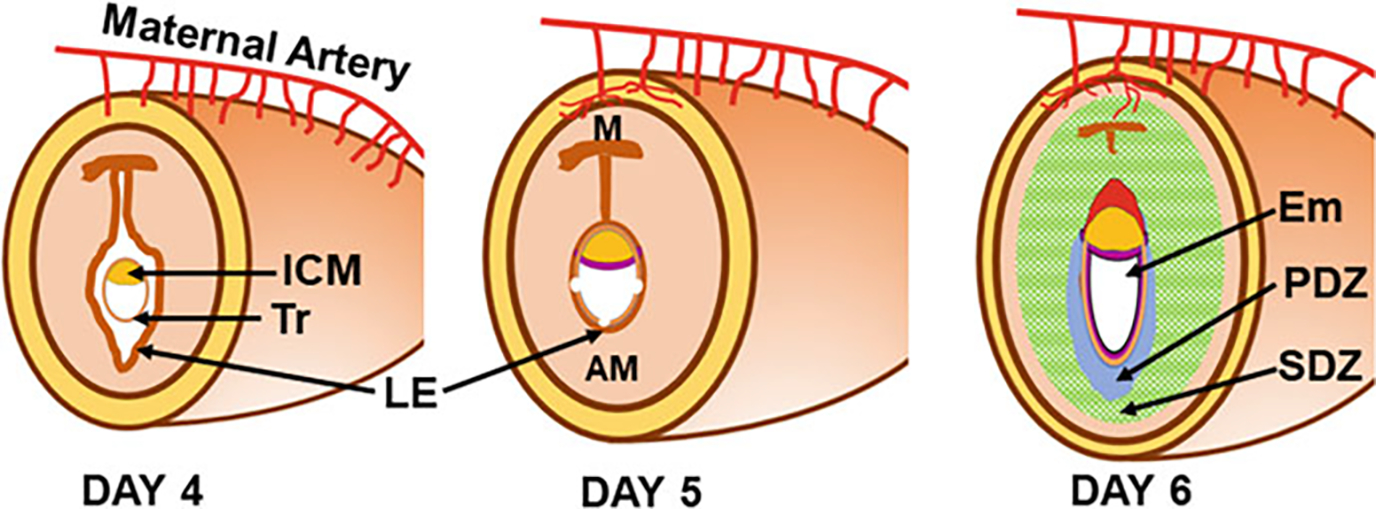
Schematic drawings of uterine and embryonic changes during early pregnancy (days 4–6 of pregnancy). Antimesometrial (AM) side, Embryo (Em), Inner cell mass (ICM), Luminal epithelium (LE), Mesometrial (M) side, Primary decidual zone (PDZ), Secondary decidual zone (SDZ), Trophectoderm (Tr)
Successful implantation of the blastocyst requires an implantation-competent blastocyst, a receptive uterus, and a mutualistic interaction between the blastocyst and the uterus. Blastocyst implantation process is divided into three steps: apposition, adhesion, and invasion (Carson et al. 2000; Zhang et al. 2013). An anomaly in any of these stages is considered to be the root cause of instantaneous pregnancy failure or placental developmental defects leading to later pregnancy complications such as placental insufficiency, preeclampsia, fetal developmental disorders, and preterm birth (Cha et al. 2012).
In general, the uterine endometrium remains receptive to blastocyst implantation for a short period which is termed “implantation window” (Paria et al. 1993). In humans, the uterus achieves receptivity in the mid-secretory phase (20–26 days) coinciding with differentiation of endometrial stromal cells into specialized decidual cells of each 28-day menstrual cycle with or without an occurrence of copulation and the presence of a blastocyst. Uterine exposure to high levels of progesterone during the luteal phase is considered critical for changes in the uterus that are needed for uterine receptivity (Simon et al. 2003). In contrast to the human, the mouse uterus exposed to only progesterone does not attain receptivity either in the lack or excess of estrogen. A successful mating during the cycle requires to attain receptivity. The preimplantation mouse uterus exposed to the augmented level of luteal progesterone achieves receptivity only when it is exposed to a small rise of circulating ovarian estrogen level around the noon of day 4 of pregnancy (Paria et al. 1993). An ideal uterine window of implantation in mice lasts only for a very short period from midnight of day 4 to early morning of day 5 (Das et al. 1994). Like uterine receptive state, blastocyst implantation-competent stage is also a determinant factor for initiation of implantation. A study using the delayed implantation model in mice has shown that activated blastocyst functions as a proinflammatory entity at the time of implantation (He et al. 2019).
The types of implantation vary from species to species based on the degree of invasion. The primates and guinea pigs exhibit interstitial type of implantation in which the blastocyst reaches the stroma disregarding the luminal epithelium (LE). In cattle, sheep, and pigs, implantation is a superficial type in which embryonic trophoblasts simply remain attached to the uterine epithelium (no penetration). In rodents such as mice and rats, implantation is an eccentric type in which a mouse blastocyst placed itself inside a cup-shaped endometrial crypt (implantation chamber). These crypts are formed on day 4 of pregnancy as a result of occasional inward folding of epithelial layer primarily in the antimesotrial (AM) side of the receptive uterus (Figs. 3 and 4) (Cha et al. 2014). Once this cocooning process ends, the blastocyst initiates contact with LE cells using some of its mural Tr cells positioning ICM and polar Tr cells of the embryonic pole towards the lumen or mesometrial (M) side. While mural Tr cells of the abembryonic pole begin initial contact with the LE in mice, polar Tr of the embryonic pole do this work in humans. However, placenta is developed exclusively from the polar Tr cells in both species. The early stage of the contact between the uterus and the blastocyst initiates transformation of the endometrial stromal cells to decidual cells only at the blastocyst–uterine contact site. As the contact and direct communication between the uterus and blastocyst gets stronger, the uterine LE cells at the site of blastocyst contact undergo apoptosis and/or entosis by the contact-making trophoblast cells to confirm direct contact with the underneath stromal/decidual cells (Fig. 4) (Li et al. 2015; Zhang and Paria 2006). Following implantation, the round blastocyst undergoes transformation to form an elongated “egg cylinder.” This occurs as a result of rapid proliferation of cells of the EP, PE, and polar Tr.
4. A Gist of Placentation
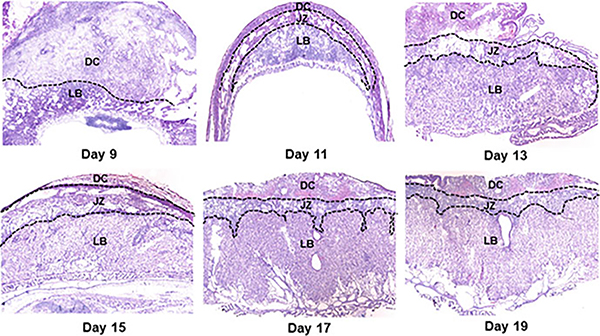
Placenta formation begins with blastocyst implantation and keeps on growing (Figs. 3, 5 and 6). After implantation occurs, the blastocyst implantation site progressively grows in size from day 5 to day 8 of pregnancy mainly due to development of the decidua (Fig. 3). Later in pregnancy, the rapid growth of the placenta and the embryo governs the size of the implantation site. A definite structure of the placenta in mice is evident by gestational days 10–11. Its development finishes by day 15 of pregnancy and maximum volume reaches by day 17 (Coan et al. 2004). It is expelled upon birth of the fetus. It has both maternal uterine and fetal components. It develops between the uterus and the fetus and acts as a caretaker of pregnancy and the fetus. Although both the humans and mice have a hemochorial type of placenta (Hafez 2017), the differences between the human and mouse placentation are considerable. Unlike the human villous placenta, the site of placental exchange in rodents in the labyrinth region has a complex arrangement of maternal and vascular channels (Hemberger et al. 2020). The mouse placenta comprises of three structures: the maternal decidua, the junctional zone (JZ), and the labyrinth (Figs. 6 and 7). The maternal decidual zone contains uterine decidual cells, maternal vasculature, glycogen trophoblast (GlyT) cells, and spiral artery-associated trophoblast giant cells (SpA-TGC). The JZ contains a p-TGC layer and a spongiotrophoblast layer consisting of spongiotrophoblasts (SpT) and GlyT cells. The labyrinth contains chorion-derived epithelial cells and allantois-derived vascular cells (Fig. 7). Here we review the current understanding of the post-implantation embryonic events that outline the operational organization of the mouse placenta.
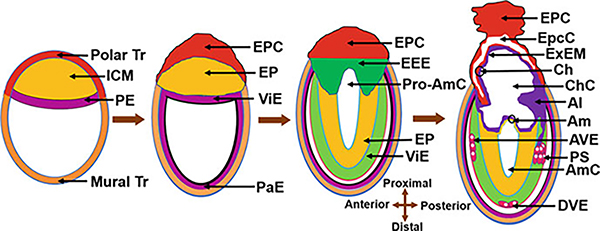
Fig. 5.
Schematic drawing of the synchronous development of the placenta and the embryo following blastocyst implantation. Allantois (Al), Amnion (Am), Amniotic cavity (AmC), Anterior visceral endoderm (AVE), Chorion (Ch), Chorionic cavity (ChC), Distal visceral endoderm (DVE), Ectoplacental cone (EPC), Ectoplacental cavity (EpcC), Extra-embryonic ectoderm (EEE), Epiblast (EP), Extra-embryonic mesoderm (ExEM), Inner cell mass (ICM), Parietal endoderm (PaE), Primitive endoderm (PE), Primitive streak (PS), Pro-amniotic cavity (Pro-AmC), Visceral endoderm (ViE), and Trophectoderm (Tr)
Fig. 6.
Photomicrographs of hematoxylin- and eosin-stained histological sections of placentae from days 9–19 of pregnancy. Decidua (DC), Junctional zone (JZ), Labyrinth (LB)
Fig. 7.
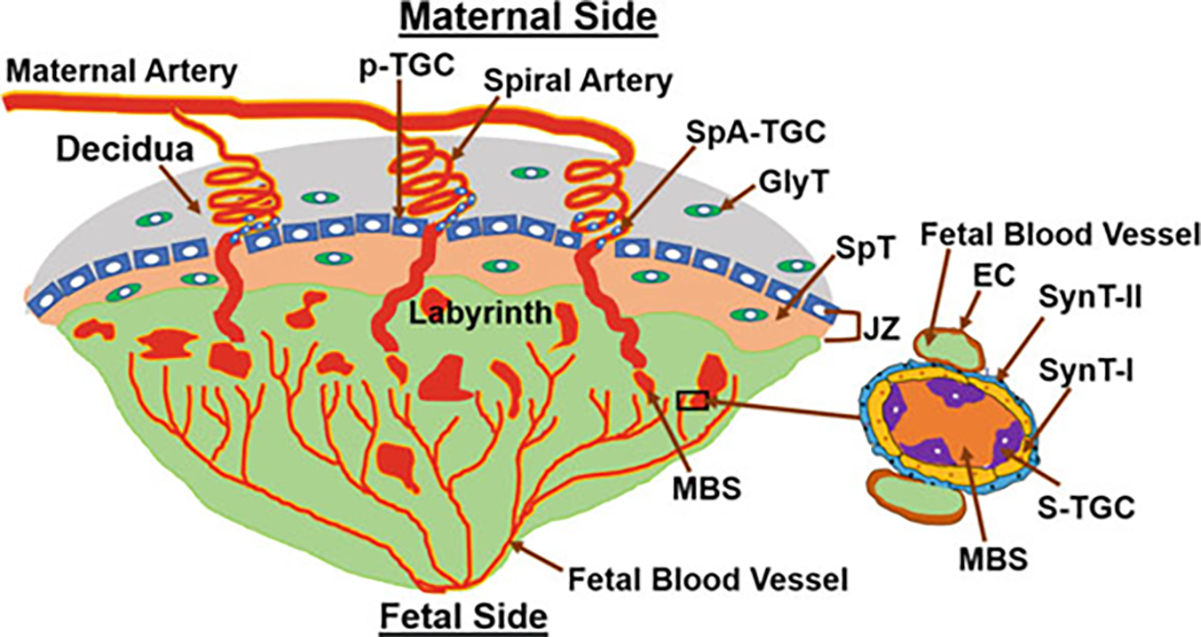
A schema of a mouse matured placenta. Endothelial cell (EC), Glycogen trophoblast (GlyT), Junctional zone (JZ), Maternal blood sinusoid (MBS), Parietal TGC (p-TGC), Sinusoidal TGC (S-TGC), Spiral artery-associated TGC (SpA-TGC), Spongiotrophoblast (SpT), Syncytotrophoblast I (SynT-I), Syncytotrophoblast II (SynT-II)
4.1. Uterine Decidua Formation for Placentation
Blastocyst attachment onto the uterine luminal epithelium in mice stimulates uterine stromal cell proliferation. Transformation of these proliferating stromal cells to decidual cells initiates shortly after the blastocyst–uterine attachment reaction (Figs. 3 and 4). It first occurs in antimesometrial (AM) stromal cells that are closest to the implantation chamber. This avascular and densely packed decidual cell region that is termed as the primary decidual zone (PDZ) is considered the first protective scaffold of the implanted embryo. The PDZ formation is fully established by day 6 (Figs. 3 and 4). Later, all other proliferating stromal cells surrounding the PDZ both at the AM and mesometrial (M) sides transform into decidual cells, forming a secondary decidual zone (SDZ) (Figs. 3 and 4). While AM decidual cells are polyploid, M decidual cells are not (Das 2009, 2010). As the pregnancy progresses from day 5 to 8, the AM decidua degenerates making space for the growing embryo, while the M decidua gradually thins out to make room for placentation, eventually forming the decidua basalis. Defective decidua formation may result in infertility or later onset of pregnancy complications such as preeclampsia, recurrent abortion, preterm birth, and intrauterine fetal growth restriction.
For a pregnancy to continue further, a solid anchoring of other placental features onto the decidua basalis and the alteration of maternal spiral arteries into soft vessels are necessary. Uterine artery at the site of placentation branches into several spiral arteries by angiogenesis (Figs. 4 and 7). Spiral arteries begin as high-resistance, low-capacity arteries and remodel to low-resistance, high-capacity structures. The key regulators of these arterial changes are leukocytes, specifically those localized in the decidua basalis (Mori et al. 2016). This vascular transformation allows the increase in maternal oxygenated blood flow needed to sustain the fetus. These spiral arteries later converge into large arterial canals that cross the JZ to the base of the chorionic plate. From there, blood flows within small maternal blood sinusoids (MBS) in the labyrinth towards the junctional zone where they converge in larger channels that return deoxygenated maternal blood. Arterial canals lack smooth muscle cells and converge at the junction to the giant cells where they open into the trophoblast-lined canals that enter the labyrinth (Fig. 7). The veins are large, thin walled, without trophoblast cells. The umbilical artery branches into arterioles that carry deoxygenated blood from the fetus to the labyrinth side closest to the junctional zone. There, they branch into capillaries. The fetal deoxygenated blood with these capillaries flows in countercurrent with the oxygenated blood from the mother.
4.2. Ectoplacental Cone (EPC) and Egg Cylinder Formation
At implantation, mural Tr cells stop proliferating, but endoreduplicate to form primary TGCs which help in the attachment of the blastocyst initially with the uterine LE and later with the stromal compartment. In contrast, polar Tr cells begin proliferating to create extra- EEE and EPC (Fig. 2). Initially, the polar Tr grows towards the EP forming the EEE, which shoves the EP in the direction of the blastocoel. Later, outside cells of the EEE proliferate and grow to form an EPC towards the uterine lumen and mesometrial side of the uterus. EPC resembles like a cap on top of the EEE (Fig. 5). When the outside cells of the EPC interlock with the uterine LE, narcosis of the epithelial cells begins, leaving Tr cells to make direct contact with the uterine decidual cells. These cells also often surround the decidual cells by sending projections. These trophoblast cells are called parietal giant cells (p-TGCs) that have the largest polyploid nuclei and highest content of nuclei among all cell-types in the EPC (Bevilacqua and Abrahamsohn 1988, 1989). P-TGCs that pass through the decidual cells establish contact with the endothelial cells and displace the cells from uterine capillary walls. On day 8 of pregnancy, the EPC fully developed (Woods et al. 2018).
Upon implantation, the EP grows, elongates, and transforms into a U-shaped columnar epithelium (primitive ectoderm) that surrounds the pro-amniotic cavity (Pro-Amc) (Fig. 5). The formation of the Pro-Amc is accomplished by cavitation. While EPC is forming, the PE that lines the luminal surface of the EP proliferates and expands to form the PiE and ViE. The cells of these two endoderms later migrate along the mural trophoblast cells. The PiE lines the luminal surface of the mural trophoblasts and the ViE envelops the EP. In a later stage of embryonic development, the ViE below the EP thickens and identifies as distal visceral endoderm (DVE). Cells from the DVE later move to the anterior region of the embryo where they become known as anterior visceral endoderm (AVE). A second subpopulation of DVE migrates and assembles at the posterior side of the embryo and form the primitive streak (PS) (Rivera-Perez et al. 2003; Rivera-Perez and Hadjantonakis 2014).
4.3. Junctional Zone (JZ) Formation
The JZ arises from the cells of the core of the EPC. It is situated between the labyrinth and the maternal decidua (Figs. 6 and 7) and functions as a source of hormones, growth factors, and energy that are required for normal growth of the placenta and the embryo. The JZ developmental defects are considered a leading cause of placental as well as fetal growth retardations. The JZ consists of two layers: A p-TGC layer that directly borders the decidua and a spongiotrophoblast (SpT) layer which consists of SpT and GlyT cells (Fig. 7). While SpT cells are dense, compact, and nonmigratory, GlyT cells are vacuolated and often migrate to the decidua through p-TGC layer (Simmons and Cross 2005). Studies have suggested that SpT and GlyT cells originate from unique cell-types of the EPC. SpT and GlyT cells also surround the central canals that pass through the labyrinth and canalassociated trophoblast giant cells (cTGCs) which lines the canal (Fig. 7). GlyT cells serve as an energy source as they store glycogen and are also source of insulin-like growth factor II (IGF-II). SpT cells along with TGCs are the main source of hormones and growth factors (Woods et al. 2018).
4.4. Labyrinth and Gastrulation Formation
Labyrinth is the largest structure of the placenta and is formed next to the JZ (Figs. 6 and 7). It is a vital but a complex structure by organization and function. The construction of labyrinth involves interactions among three structures: the EPC, the chorion, and the allantois (Fig. 5). The EPC and chorion are formed from trophoblast cells, whereas the allantois is derived from extraembryonic mesoderm which originates from the epiblast.
The PS formation is the first morphological landmark of gastrulation (E 6.5) and labyrinth construction (Fig. 5). At gastrulation, the PS forms on the side of the embryo opposite to the AVE, denoting AVE as the anterior pole of the body axis and PS as the posterior pole of the embryo (Rossant and Tam 2009). The PS is the site where cells undergo an epithelial-to-mesenchymal transition. As the PS elongates, epiblast cells that are on the inside of the cup ingress up in the streak. The EP cells that move through the streak become mesoendoderm which are the precursors not only for embryonic mesoderm and extraembryonic mesoderm (ExEM) of the chorion (Ch), amnion (Am), yok sac and allantois (Al) but also for amniotic ectoderm as well as embryonic ectoderm and endoderm (Fig. 2). After they exit the primitive streak, the cells create a wave of mesoendoderm that expands between the visceral endoderm and epiblast (Woods et al. 2018).
Chorion Formation
During gastrulation, the EEE folds inside the Pro-AmC from both sites and fuses together forming the ectoplacental cavity (EpcC) (Fig. 5). At the same time, a chorionic or exocoelomic cavity (ChC) is formed when extraembryonic mesoderm (ExEM) grew from the ventral and dorsal regions of the allantois and reaches the anterior margin of the egg cylinder through the Pro-AmC. The allantois initially arises from the PS as a bud. Later it grows and expands within the exocoelomic cavity. The cavity that remains in contact with the EP is called the amniotic cavity (AmC). Proximal ExEM normally grows along the base of the EEE of the ectoplacental cavity and together form the chorion (ChC). The area that contains these two layers is called the chorionic plate. Distal ExEM when it comes in contact with a layer of embryonic ectoderm form the amnion (Am) that lies in between the ChC and AmC. At day 7 of pregnancy, extraembryonic mesoderm cells of the allantois proliferate and grow inside the chorionic cavity reaching the chorion. This eliminates the EpcC and brings the chorionic plate in contact with the EEC. Chorionic plate now is a multilayered structure and becomes known as the chorio-allantoic plate (Simmons et al. 2008).
Allantois (Al) and Yolk Sac
The AL forms the fetal vascular compartment of the placenta and umbilical cord in mice (Arora and Papaioannou 2012). It arises as a bud of mesoderm from the primitive streak and grows toward the chorion through the exocoelomic cavity. The growing allantois consists of an outer layer of mesothelium that envelops a mesenchymal core (Fig. 5). Endothelial cell (EC) generation starts in the allantois from the mesenchyme. Whether hematopoietic cells arise from the endothelium or its mesenchyme is still a matter of debate. However, studies suggest that hematopoietic cells in placenta emerge via endothelial to hematopoietic transition. The ECs initially form a primary vascular plexus and connect with the dorsal aortae of the embryo prior to chorio-allantois fusion. The primary plexus then remodels to form the central vessel by a process called vasculogenesis and later the umbilical artery and vein at day 10 of pregnancy (Azevedo and Pelajo-Machado 2018). The artery and vein then invade chorion by angiogenesis forming the fetal vascular components of the labyrinth. After chorio-allantoic attachment, the chorion begins to form the villi across the chorionic surface creating spaces into which the fetal blood vessels grow from the allantois (Downs 1998; Downs and Gardner 1995).
The maternal blood spaces within the labyrinth are separated by three layers of trophoblast cells and a layer of fetal endothelial cells. The three layers of trophoblast cells are a layer of mononuclear sinusoidal trophoblast giant cells (S-TGCs) that surround maternal blood sinusoids followed by two layers of syncytiotrophoblasts, SynT-I, and SynT-II in contact with fetal vessels on the opposite site (Fig. 7). Syncytiotrophoblast cell layers are multinucleated and form as a result of trophoblast cell–cell fusion. SynT-II remains in contact with fetal endothelial cells. Thus, maternal blood comes in direct contact with trophoblast cells rather than endothelial cells of the vasculature in mice (Watson and Cross 2005).
Yolk sac is composed of two layers: The ViE and the ExEM. The difference between the mouse and human yolk sacks is that while the mouse yolk sac envelops the embryo, human yolk sac remains attached to, but does not envelop the embryo (Freyer and Renfree 2009). It has been shown that mouse yolk sac contains in vivo colony forming cells capable of producing granulocytic, megakaryocytic, and erythroid spleen colonies (Garcia and Larina 2014; Palis and Koniski 2005; Yamane 2018). Thus, yolk sac may be an exporter of hematopoietic stem cells which subsequently colonize other hematopoietic sites. It has been long known that yolk sac produces cholesterol, an essential component of the membrane and steroid synthesis (Woollett 2011). Furthermore, the endoderm layer of the yolk sac expresses growth factors and transcription factors that are required for vasculogenesis and angiogenesis (Freyer and Renfree 2009).
5. Conclusion
As the placenta is a principal transient organ of nutrient supply to the growing embryo during pregnancy, adequate placental function is instrumental for developmental progression of the intrauterine embryo. Studies have established that defects or delay in implantation, uterine stromal cell decidualization or uterine vascular remodeling at the implantation site lead to a spectrum of pregnancy complications such as poor placentation, embryonic demise, or abnormal embryonic growth and development.
The mouse is the leading model system for studying and understanding the basic structural and functional aspects of the placenta due to ethical constraints and inaccessibility of very early human implantation tissues. In this regard, gene expression and manipulation studies in mice have provided a wealth of information. The cell lineage tracing studies established that the majority defining structures of the placenta is originated from trophoblast cells of the blastocyst, but endothelial cells that make the fetal placental vasculature is aroused from the ICM of the blastocyst. Phenotypic analysis of global as well as conditional gene knockout mice has provided further information about key roles of genes and gene networks behind the complex morphological and molecular events that occur during placenta formation. More recently stem cell technologies have also begun to shed new lights on the origin of placenta cell lineages. So far thousands of genes that cause either embryonic lethality and/or placental defects have been identified. However, it is also possible that there are hundreds if not thousands of genes that influence placental developmental and functional defects remain to be identified. Taken together, gaining insights into genes and gene networks that affect implantation, decidualization, and placental structural and functional defects using the mouse model will promote the study of human pregnancy complication involving placental and embryonic developmental defects.
Acknowledgement
Research in the authors’ laboratory is supported by R01 HD094946 from the Eunice Kennedy Shriver National Institute of Child Health and Human Development/National Institute of Health.
Abbreviations
- Al
Allantois
- Am
Amnion
- AM
Antimesometrial side
- Amc
Amniotic cavity
- AVE
Anterior visceral endoderm
- BC
Blastocoel
- Bl
Blastocyst
- Ch
Chorion
- ChC
Chorionic cavity
- CM
Circular muscle
- cTGCs
Canal-associated trophoblast giant cells
- DC
Decidua
- DVE
Distal visceral endoderm
- EC
Endothelial cell
- ECC
Exocoelomic cavity
- EEE
Extra-embryonic ectoderm
- EEEn
Extra-embryonic endoderm
- Em
Embryo
- EP
Epiblast
- EPC
Ectoplacental cone
- EpcC
Ectoplacental cavity
- ExEM
Extra-embryonic mesoderm
- GlyT
Glycogen trophoblast
- ICM
Inner cell mass
- IGF-II
Insulin-like growth factor II
- JZ
Junctional zone
- LE
Luminal epithelium
- LM
Longitudinal muscle
- M
Mesometrial side
- MBS
Maternal blood sinusoid
- PaE
Parietal endoderm
- PDZ
Primary decidual zone
- PE
Primitive endoderm
- Pro-AmC
Pro-amniotic cavity
- PS
Primitive streak
- p-TGCs
Parietal giant cells
- SDZ
Secondary decidual zone
- SpA-TGC
Spiral artery-associated trophoblast giant cells
- SpT
Spongiotrophoblast
- S-TGCs
Sinusoidal trophoblast giant cells
- SynT-I
Syncytotrophoblast I
- SynT-II
Syncytotrophoblast II
- TGCs
Trophoblast giant cells
- Tr
Trophectoderm
- ViE
Visceral endoderm
- ZP
Zona pellucida
References
- Abe KI, Funaya S, Tsukioka D, Kawamura M, Suzuki Y, Suzuki MG, Schultz RM, Aoki F (2018) Minor zygotic gene activation is essential for mouse preimplantation development. Proc Natl Acad Sci U S A 115(29):E6780–E6788. Available from: PM:29967139 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arora R, Papaioannou VE (2012) The murine allantois: a model system for the study of blood vessel formation. Blood 120(13):2562–2572. Available from: PM:22855605 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Azevedo PN, Pelajo-Machado M (2018) Mechanism of hematopoiesis and vasculogenesis in mouse placenta. Placenta 69:140–145. Available from: PM:29680159 [DOI] [PubMed] [Google Scholar]
- Bauer ST, Bonanno C (2009) Abnormal placentation. Semin Perinatol 33(2):88–96. Available from: PM:19324237 [DOI] [PubMed] [Google Scholar]
- Bevilacqua EM, Abrahamsohn PA (1988) Ultrastructure of trophoblast giant cell transformation during the invasive stage of implantation of the mouse embryo. J Morphol 198(3):341–351. Available from: PM:3221406 [DOI] [PubMed] [Google Scholar]
- Bevilacqua EM, Abrahamsohn PA (1989) Trophoblast invasion during implantation of the mouse embryo. Arch Biol Med Exp (Santiago) 22(2):107–118. Available from: PM:2619314 [PubMed] [Google Scholar]
- Brown LD, Hay WW Jr (2016) Impact of placental insufficiency on fetal skeletal muscle growth. Mol Cell Endocrinol 435:69–77. Available from: PM:26994511 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Browne JC (1963) Placental insufficiency. Scott Med J 8:459–465. Available from: PM:14089221 [DOI] [PubMed] [Google Scholar]
- Burton GJ, Fowden AL, Thornburg KL (2016) Placental origins of chronic disease. Physiol Rev 96 (4):1509–1565. Available from: PM:27604528 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carson DD, Bagchi I, Dey SK, Enders AC, Fazleabas AT, Lessey BA, Yoshinaga K (2000) Embryo implantation. Dev Biol 223(2):217–237. Available from: PM:10882512 [DOI] [PubMed] [Google Scholar]
- Cha J, Sun X, Dey SK (2012) Mechanisms of implantation: strategies for successful pregnancy. Nat Med 18(12):1754–1767. Available from: PM:23223073 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cha J, Bartos A, Park C, Sun X, Li Y, Cha SW, Ajima R, Ho HY, Yamaguchi TP, Dey SK (2014) Appropriate crypt formation in the uterus for embryo homing and implantation requires Wnt5a-ROR signaling. Cell Rep 8(2):382–392. Available from: PM:25043182 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chazaud C, Yamanaka Y (2016) Lineage specification in the mouse preimplantation embryo. Development 143(7):1063–1074. Available from: PM:27048685 [DOI] [PubMed] [Google Scholar]
- Coan PM, Ferguson-Smith AC, Burton GJ (2004) Developmental dynamics of the definitive mouse placenta assessed by stereology. Biol Reprod 70(6):1806–1813. Available from: PM:14973263 [DOI] [PubMed] [Google Scholar]
- Das SK (2009) Cell cycle regulatory control for uterine stromal cell decidualization in implantation. Reproduction 137(6):889–899. Available from: PM:19307426 [DOI] [PubMed] [Google Scholar]
- Das SK (2010) Regional development of uterine decidualization: molecular signaling by Hoxa-10. Mol Reprod Dev 77(5):387–396. Available from: PM:19921737 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Das SK, Wang XN, Paria BC, Damm D, Abraham JA, Klagsbrun M, Andrews GK, Dey SK (1994) Heparin-binding EGF-like growth factor gene is induced in the mouse uterus temporally by the blastocyst solely at the site of its apposition: a possible ligand for interaction with blastocyst EGF-receptor in implantation. Development 120(5):1071–1083. Available from: PM:8026321 [DOI] [PubMed] [Google Scholar]
- Downs KM (1998) The murine allantois. Curr Top Dev Biol 39:1–33. Available from: PM:9475996 [DOI] [PubMed] [Google Scholar]
- Downs KM, Gardner RL (1995) An investigation into early placental ontogeny: allantoic attachment to the chorion is selective and developmentally regulated. Development 121(2):407–416. Available from: PM:7768182 [DOI] [PubMed] [Google Scholar]
- Freyer C, Renfree MB (2009) The mammalian yolk sac placenta. J Exp Zool B Mol Dev Evol 312 (6):545–554. Available from: PM:18985616 [DOI] [PubMed] [Google Scholar]
- Garcia MD, Larina IV (2014) Vascular development and hemodynamic force in the mouse yolk sac. Front Physiol 5:308. Available from: PM:25191274 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo S, Cui X, Jiang X, Duo S, Li S, Gao F, Wang H (2020) Tracing the origin of the placental trophoblast cells in mouse embryo developmentdagger. Biol Reprod 102(3):598–606. Available from: PM:31621828 [DOI] [PubMed] [Google Scholar]
- Hafez S (2017) Comparative placental anatomy: divergent structures serving a common purpose. Prog Mol Biol Transl Sci 145:1–28. Available from: PM:28110748 [DOI] [PubMed] [Google Scholar]
- He B, Zhang H, Wang J, Liu M, Sun Y, Guo C, Lu J, Wang H, Kong S (2019) Blastocyst activation engenders transcriptome reprogram affecting X-chromosome reactivation and inflammatory trigger of implantation. Proc Natl Acad Sci U S A 116(33):16621–16630. Available from: PM:31346081 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hemberger M, Hanna CW, Dean W (2020) Mechanisms of early placental development in mouse and humans. Nat Rev Genet 21(1):27–43. Available from: PM:31534202 [DOI] [PubMed] [Google Scholar]
- Ikawa M, Inoue N, Benham AM, Okabe M (2010) Fertilization: a sperm’s journey to and interaction with the oocyte. J Clin Invest 120(4):984–994. Available from: PM:20364096 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kollmann M, Gaulhofer J, Lang U, Klaritsch P (2016) Placenta praevia: incidence, risk factors and outcome. J Matern Fetal Neonatal Med 29(9):1395–1398. Available from: PM:26043298 [DOI] [PubMed] [Google Scholar]
- Li Y, Sun X, Dey SK (2015) Entosis allows timely elimination of the luminal epithelial barrier for embryo implantation. Cell Rep 11(3):358–365. Available from: PM:25865893 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mori M, Bogdan A, Balassa T, Csabai T, Szekeres-Bartho J (2016) The decidua-the maternal bed embracing the embryo-maintains the pregnancy. Semin Immunopathol 38(6):635–649. Available from: PM:27287066 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oyelese Y, Ananth CV (2006) Placental abruption. Obstet Gynecol 108(4):1005–1016. Available from: PM:17012465 [DOI] [PubMed] [Google Scholar]
- Oyelese Y, Smulian JC (2006) Placenta previa, placenta accreta, and vasa previa. Obstet Gynecol 107(4):927–941. Available from: PM:16582134 [DOI] [PubMed] [Google Scholar]
- Palis J, Koniski A (2005) Analysis of hematopoietic progenitors in the mouse embryo. Methods Mol Med 105:289–302. Available from: PM:15492402 [DOI] [PubMed] [Google Scholar]
- Paria BC, Huet-Hudson YM, Dey SK (1993) Blastocyst’s state of activity determines the “window” of implantation in the receptive mouse uterus. Proc Natl Acad Sci U S A 90(21):10159–10162. Available from: PM:8234270 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phipps EA, Thadhani R, Benzing T, Karumanchi SA (2019) Pre-eclampsia: pathogenesis, novel diagnostics and therapies. Nat Rev Nephrol 15(5):275–289. Available from: PM:30792480 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Posfai E, Rovic I, Jurisicova A (2019) The mammalian embryo’s first agenda: making trophectoderm. Int J Dev Biol 63(3–4-5):157–170. Available from: PM:31058294 [DOI] [PubMed] [Google Scholar]
- Rivera-Perez JA, Hadjantonakis AK (2014) The dynamics of morphogenesis in the early mouse embryo. Cold Spring Harb Perspect Biol 7(11):a015867. Available from: PM:24968703 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rivera-Perez JA, Mager J, Magnuson T (2003) Dynamic morphogenetic events characterize the mouse visceral endoderm. Dev Biol 261(2):470–487. Available from: PM:14499654 [DOI] [PubMed] [Google Scholar]
- Rossant J, Cross JC (2001) Placental development: lessons from mouse mutants. Nat Rev Genet 2 (7):538–548. Available from: PM:11433360 [DOI] [PubMed] [Google Scholar]
- Rossant J, Tam PP (2009) Blastocyst lineage formation, early embryonic asymmetries and axis patterning in the mouse. Development 136(5):701–713. Available from: PM:19201946 [DOI] [PubMed] [Google Scholar]
- Simmons DG, Cross JC (2005) Determinants of trophoblast lineage and cell subtype specification in the mouse placenta. Dev Biol 284(1):12–24. Available from: PM:15963972 [DOI] [PubMed] [Google Scholar]
- Simmons DG, Natale DR, Begay V, Hughes M, Leutz A, Cross JC (2008) Early patterning of the chorion leads to the trilaminar trophoblast cell structure in the placental labyrinth. Development 135(12):2083–2091. Available from: PM:18448564 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simon C, Dominguez F, Valbuena D, Pellicer A (2003) The role of estrogen in uterine receptivity and blastocyst implantation. Trends Endocrinol Metab 14(5):197–199. Available from: PM:12826321 [DOI] [PubMed] [Google Scholar]
- Watson ED, Cross JC (2005) Development of structures and transport functions in the mouse placenta. Physiology (Bethesda) 20:180–193. Available from: PM:15888575 [DOI] [PubMed] [Google Scholar]
- Woods L, Perez-Garcia V, Hemberger M (2018) Regulation of placental development and its impact on fetal growth-new insights from mouse models. Front Endocrinol (Lausanne) 9:570. Available from: PM:30319550 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woollett LA (2011) Review: transport of maternal cholesterol to the fetal circulation. Placenta 32 (Suppl 2):S218–S221. Available from: PM:21300403 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamane T (2018) Mouse yolk sac hematopoiesis. Front Cell Dev Biol 6:80. Available from: PM:30079337 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshinaga K (2013) A sequence of events in the uterus prior to implantation in the mouse. J Assist Reprod Genet 30(8):1017–1022. Available from: PM:24052329 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Q, Paria BC (2006) Importance of uterine cell death, renewal, and their hormonal regulation in hamsters that show progesterone-dependent implantation. Endocrinology 147(5):2215–2227. Available from: PM:16469810 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang S, Lin H, Kong S, Wang S, Wang H, Wang H, Armant DR (2013) Physiological and molecular determinants of embryo implantation. Mol Aspects Med 34(5):939–980. Available from: PM:23290997 [DOI] [PMC free article] [PubMed] [Google Scholar]