Figure 5. Inhibiting Calcineurin/NFAT or Cardiac Myosin Mitigates Development of the HCM Phenotype in Proband iPSC-Derived Cardiomyocytes.
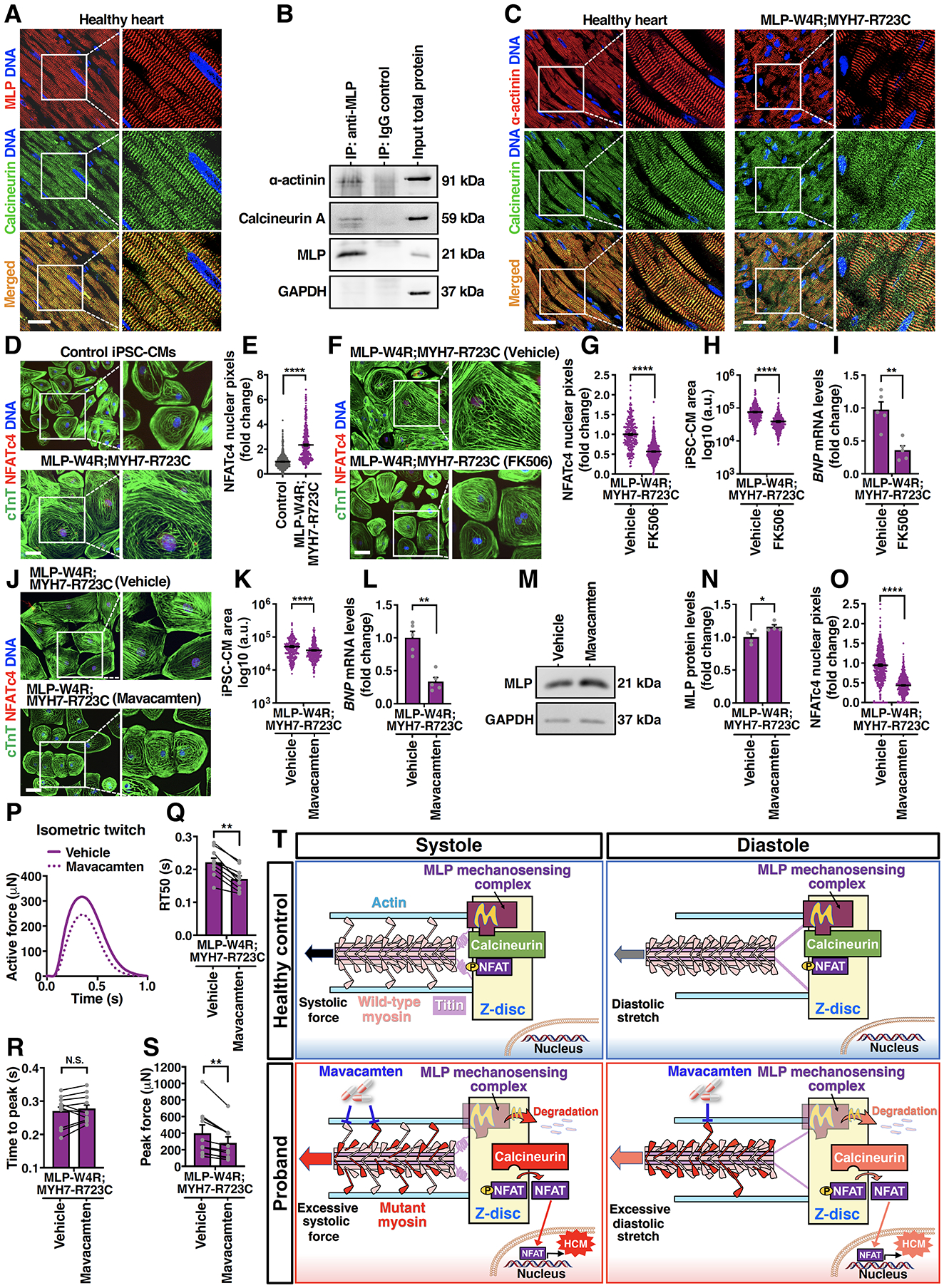
A, Immunostaining of calcineurin (green) and MLP (red) in the young adult healthy heart tissue. DNA was counterstained by DAPI. Scale bar, 50 μm. B, A representative immunoblot showing co-immunoprecipitation of MLP, calcineurin, and α-actinin in day 35 control iPSC-CMs. C, Immunostaining of calcineurin (green) and α-actinin (red) in the young adult healthy heart tissue and MLP-W4R;MYH7-R723C proband heart tissue. Scale bar, 50 μm. D, Immunostaining of NFATc4 (red) and cTnT (green) in day 35 control and MLP-W4R;MYH7-R723C iPSC-CMs. DNA was counterstained by DAPI. Scale bar, 100 μm. E, Quantification of NFATc4 nuclear signals in panel D (two-tailed unpaired Student’s t test). Nuclear NFATc4 pixels (gray value) were quantified by ImageJ from three independent cardiomyocyte differentiation batches (≥100 cells/batch). F, Immunostaining of NFATc4 (red) and cTnT (green) in MLP-W4R;MYH7-R723C iPSC-CMs treated with DMSO (vehicle) or 0.5 μg/mL calcineurin inhibitor FK506. Treatment was started on day 25 of cardiac differentiation for 4 days (G and H) and 24 hours (I). Scale bar, 100 μm. G-I, Quantification of NFATc4 nuclear signals (G), cell area (H), and BNP gene expression (I) in DMSO- or FK506-treated MLP-W4R;MYH7-R723C iPSC-CMs. A two-tailed unpaired Student’s t test was performed for nuclear NFATc4 and cell area analyses from three independent cardiomyocyte differentiation batches (≥100 cells/batch). A two-tailed unpaired Mann-Whitney U test was used for BNP gene analysis (n=5 independent cardiomyocyte differentiation batches per group; normalized to GAPDH). J, Immunostaining of NFATc4 (red) and cTnT (green) in MLP-W4R;MYH7-R723C iPSC-CMs treated with DMSO (vehicle) or 0.5 μM cardiac myosin ATPase inhibitor mavacamten. Treatment was started on day 25 of cardiac differentiation for four days (J, K, M, N, and O) and 24 hours (L). Scale bar, 100 μm. K-L, Quantification of cell area (K) and BNP gene expression (L) in DMSO- or mavacamten-treated MLP-W4R;MYH7-R723C iPSC-CMs. A two-tailed unpaired Student’s t test was performed for cell area analysis from three independent cardiomyocyte differentiation batches (≥100 cells/batch). A two-tailed unpaired Mann-Whitney U test was used for BNP gene analysis (n=5 independent cardiomyocyte differentiation batches per group; normalized to GAPDH). M, A representative western blot of MLP expression in vehicle- and mavacamten-treated MLP-W4R;MYH7-R723C iPSC-CMs. N, Quantification of MLP protein levels in panel M. A Mann-Whitney U test was used for MLP protein analysis (n=4 independent cardiomyocyte differentiation batches per group; normalized to GAPDH). O, Quantification of NFATc4 nuclear signals in vehicle and mavacamten-treated MLP-W4R;MYH7-R723C iPSC-CMs in panel J. A two-tailed unpaired Student’s t test was performed based on three independent cardiomyocyte differentiation batches (≥100 cells/batch). P, Representative paired measurement of proband MLP-W4R;MYH7-R723C iPSC-CM-derived EHTs treated with DMSO (vehicle) or 0.5 μM mavacamten to steady state (30 minutes). Q-S, Quantification of biomechanical properties including RT50 (Q), time to peak (R), and peak force (S) (ten EHTs generated from three independent proband cardiomyocyte differentiation batches). A two-tailed paired Mann-Whitney U test was used for analysis between two groups. T, Schematic of the proposed working model. Mechanical force is transmitted into the Z-disc primarily through pulling actin by myosin heads via actomyosin crossbridges during systolic contraction. Titin is the main mechanical element transferring load into the Z-disc during diastolic stretch, with very little mechanical input through actin due to the detachment of myosins from actin. MLP mechanosensing complex may keep calcineurin/NFAT signaling in check under normal systolic and diastolic conditions. In contrast, the MYH7-R723C mutation in the proband resulted in abnormally higher systolic force transmitted into the Z-disc due to more crossbridge formation. Additionally, residual mutant actomyosin crossbridges due to delayed relaxation in the proband could lead to elevated diastolic Z-disc stretching. Consequently, MLP stretch-sensing machinery would likely be sensitized, leading to MLP degradation and subsequent calcineurin-NFAT hypertrophic signaling. Importantly, treatment of proband CMs with mavacamten, a cardiac myosin ATPase inhibitor, could potentially normalize systolic force generation and diastolic stretching, resulting in a restored MLP level and rescue from HCM defects. See the main text for details. Each data point represents a single iPSC-CM (E, G, H, K, and O), sample generated from a batch of iPSC-CMs (I, L, and N), or EHT (Q-S) derived from at least three independent cardiomyocyte differentiation batches. All data are presented as mean ± S.E.M; *p<0.05; **p<0.01; ***p<0.001; ****p<0.0001; N.S: not significant.
