Abstract
SARS-CoV-2 RNA quantification in wastewater is an important tool for monitoring the prevalence of COVID-19 disease on a community scale which complements case-based surveillance systems. As novel variants of concern (VOCs) emerge there is also a need to identify the primary circulating variants in a community, accomplished to date by sequencing clinical samples. Quantifying variants in wastewater offers a cost-effective means to augment these sequencing efforts. In this study, SARS-CoV-2 N1 RNA concentrations and daily loadings were determined and compared to case-based data collected as part of a national surveillance programme to determine the validity of wastewater surveillance to monitor infection spread in the greater Dublin area. Further, sequencing of clinical samples was conducted to determine the primary SARS-CoV-2 lineages circulating in Dublin. Finally, digital PCR was employed to determine whether SARS-CoV-2 VOCs, Alpha and Delta, were quantifiable from wastewater. No lead or lag time was observed between SARS-CoV-2 wastewater and case-based data and SARS-CoV-2 trends in Dublin wastewater significantly correlated with the notification of confirmed cases through case-based surveillance preceding collection with a 5-day average. This demonstrates that viral RNA in Dublin's wastewater mirrors the spread of infection in the community. Clinical sequence data demonstrated that increased COVID-19 cases during Ireland's third wave coincided with the introduction of the Alpha variant, while the fourth wave coincided with increased prevalence of the Delta variant. Interestingly, the Alpha variant was detected in Dublin wastewater prior to the first genome being sequenced from clinical samples, while the Delta variant was identified at the same time in clinical and wastewater samples. This work demonstrates the validity of wastewater surveillance for monitoring SARS-CoV-2 infections and also highlights its effectiveness in identifying circulating variants which may prove useful when sequencing capacity is limited.
Keywords: SARS-CoV-2, Digital PCR, Wastewater surveillance, Genomic surveillance, Variants
Graphical abstract
1. Introduction
The emergence and spread of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) has created an unprecedented global public health emergency (Zhou et al., 2020). Infection symptoms can range from fever and lethargy to respiratory impairment in which ventilation is required. Thus, severe burdens both in mortality and morbidity and, also, serious socioeconomic consequences have resulted (Leal Filho et al., 2020). Although clinical diagnostic testing and close contact tracing continue in many countries, current efforts are also focused on mass vaccination campaigns and developing effective treatments including therapeutic monoclonal antibodies (Twomey et al., 2020). Vaccines and most passive antibody cocktail therapies target the spike protein (S), a homotrimeric viral envelope protein that mediates host-cell entry via the cellular receptor angiotensin-converting enzyme 2 (ACE2) (Baum et al., 2020; Lan et al., 2020). Amino acid substitutions in the S protein have effects on protein folding and immune evasion by creating mutants unrecognised by antibodies (Greaney et al., 2021; Harvey et al., 2021). Therefore, the circulation of variants with mutations in S could have a significant impact upon the protective efficacy of prophylactic vaccines and passive antibody-based treatments.
Among the variants with mutations in S, variants Alpha and Delta have been characterized with enhanced transmissibility and increased odds of causing hospitalisations compared with preceding variants (Funk et al., 2021). The Alpha variant was initially distinguished by the S substitution N501Y that has been shown to strengthen the binding of the virus receptor binding domain (RBD) to ACE2 (Tian et al., 2021). The S:L452R associated with the Delta variant has been shown to increase the viral infectivity, promote viral replication and improve cellular immunity evasion (Motozono et al., 2021). The introduction of both variants to countries has been followed by surges in the number of positive cases.
In addition to being detected in sputum and saliva, SARS-CoV-2 RNA is shed in the faeces of up to 67% of infected patients and is also present in the faeces of asymptomatic individuals (Chen et al., 2020; Han et al., 2020). The presence of SARS-CoV-2 RNA in faeces indicated that wastewater could be used to monitor infection rates in a treatment plant's catchment population. Indeed, from the outset of the COVID-19 pandemic wastewater monitoring had already commenced in a number of laboratories around the world (Ahmed et al., 2020; La Rosa et al., 2020). Among the first was Medema et al. who identified SARS-CoV-2 RNA in wastewater in The Netherlands prior to the first clinical cases (Medema et al., 2020). According to the University of California, Merced SARS-CoV-2 wastewater monitoring dashboard, there are at least 66 countries engaged in SARS-CoV-2 wastewater surveillance with some, including Ireland, conducting national-scale monitoring.
Wastewater surveillance programmes offer a potentially cost-effective and non-invasive means to monitor pathogens circulating in communities or trends in rates of COVID-19 in the population that complements national testing efforts (Feng et al., 2021; LaTurner et al., 2021). This is because a single wastewater sample represents a pooled sample from the population contributing to the wastewater treatment plant (WWTP) which can be thousands or millions of people (Shah et al., 2022). Moreover, typically only symptomatic individuals or close contacts of SARS-CoV-2 positive patients present for clinical testing so a large proportion of infected people may go undiagnosed, particularly when testing capacity is low (Sharif et al., 2021). Wastewater surveillance is not impacted by this bias as infected individuals in a catchment will contribute passively to the wastewater system whether they are symptomatic or not (Hata and Honda, 2020). This aspect of wastewater surveillance will be beneficial as infection trends can continue to be monitored as vaccination rates increase which will result in an increased proportion of asymptomatic infections (Polack et al., 2020). Monitoring wastewater for SARS-CoV-2 variants also offers a means to augment the sequencing of clinical samples which is expensive and not possible for every positive case due to limitations on input viral load, laboratory capacity and cost (Viveros et al., 2021).
The greater Dublin area is served by a WWTP that receives wastewater from 40% of Ireland's population. Thus, the aim of this study was to determine if SARS-CoV-2 RNA levels in Dublin wastewater mirror trends determined through a case-based surveillance system in Dublin. Furthermore, wastewater samples were analysed for the presence of the Alpha and Delta variants to identify if the presence of these variants in wastewater aligned with their first identification in clinical sequence data in the catchment area.
2. Methods
2.1. Wastewater sample collection
Between June 2020 and August 2021, 24-h composite wastewater influent samples (n = 99) were collected twice per week from the Ringsend WWTP that receives influent from the greater Dublin area (Table S1). Composite samples were time-weighted with subsamples collected every hour. The Ringsend WWTP is the largest WWTP in the Republic of Ireland, receiving a collected load of approximately 1.9 million population equivalents. The flow rate (m3/day) for the Ringsend WWTP on the day of sampling was obtained from Irish Water.
2.2. Composite wastewater influent concentration and nucleic acid extraction
Composite wastewater influent samples were concentrated using 100 kDa Centricon Plus-70 filters (Merck, Germany). Briefly, the filter devices were washed by filling with 70 ml of distilled water and centrifuging at 3200g for 5 min. Composite wastewater influent samples (200–250 ml) were centrifuged at 3200g to remove solids. The resulting supernatants were then passed through the filters in 70 ml aliquots by centrifuging at 3200g for 15–40 min at a time. Approximately 500 μl of concentrated wastewater was recovered by inverting the filter into a collection cup and centrifuging at 1000g for 2 min. RNA was extracted from 250 μl of the wastewater influent concentrates using the RNeasy PowerMicrobiome Kit (Qiagen, Germany) according to the manufacturer's protocols. This protocol was validated using 50 ml of SARS-CoV-2 negative sewage spiked with heat inactivated SARS-CoV-2 (3 × 107 gc/ml). The recovery range was found to be between 50% and 94.7% (Table S2).
2.3. qPCR assays to quantify SARS-CoV-2 RNA
Reverse Transcription-quantitative PCR (RT-qPCR) assays were performed on the Roche LightCycler 96 platform (Roche Diagnostics, Germany). SARS-CoV-2 RT-qPCR assays were conducted using the LightCycler Multiplex RNA Virus Master (Roche Diagnostics, Germany) according to the manufacturer's guidelines in 20 μl volumes, containing 0.5 μM each of forward and reverse primers, 0.125 μM probe, water and 5 μl sample RNA (or 5 μl plasmid standard DNA). The primer details and thermocycling conditions are listed in Table S3. All samples, negative controls and extraction blanks were analysed in duplicate, while standards were included in triplicate in each 96-well plate. The nCoV-CDC-Control plasmid was used a standard for N1 qPCR assays (CDC, 2020; Lu et al., 2020). Results were expressed as gene copies (gc)/100 ml. Reaction efficiencies for each assay were determined using the E = 10(1/slope) -1 equation (Rutledge and Côté, 2003). The limit of detection (LOD) was determined as the lowest concentration of DNA detected in 95% or more of replicates and the limit of quantification (LOQ) was determined as the lowest concentration of DNA quantified within 0.5 standard deviations of the log10 concentration (Table S3) (Rutledge and Stewart, 2008).
2.4. SARS-CoV-2 whole genome sequencing from clinical samples
Virus genome sequencing in Ireland was performed in the context of enhanced surveillance in support and to inform public health investigations, so ethical approval was not required. For this study, only sequences from samples collected from cases resident in County Dublin between June 2020 and August 2021 and made publicly available in GISAID (www.gisaid.org) were used. SARS-CoV-2 whole genome sequences were generated from clinical samples with Ct values ≤25 using the tiled amplicon approach described by Freed et al. and nanopore sequencing on the GridION platform (Oxford Nanopore Technologies, UK) (Freed et al., 2020). Briefly, 8 μl viral RNA was reverse transcribed with 2 μl of LunaScript (NEB, USA) and cDNA was amplified using two separate primer pools to generate overlapping 1200 bp tiled amplicons by amplification with Q5 Hot Start High-Fidelity DNA polymerase (NEB, USA). PCR reactions were subsequently combined, and barcodes were assigned using the rapid barcoding kit (SQK-RBK110.96; ONT, UK) as described by the manufacturer. Barcoded samples were pooled in a 2 ml Eppendorf DNA LoBind tube then incubated with SPRI magnetic beads for 10 min on a HulaMixer (Invitrogen, UK). Samples were magnetically separated and then washed twice with 80% ethanol and eluted in buffer EB. Attachment of sequencing adapters of 800 ng of cleaved cDNA was performed by addition of RAP-F and incubation at room temperature for 5 min then placed on ice. Libraries were sequenced on FLO-MIN106D flow cells on the GridION platform (ONT, UK).
2.5. Phylogenetic analysis of sequences from contemporaneous samples
The lineages of the sequences were assigned using PANGOLIN with the pangoLEARN version 2021-09-28 and grouped according to the variant and super lineage names associated to each lineage (Rambaut et al., 2020a; O’Toole et al., 2021). To visualize the phylogenetic relation among sequences, a phylogenetic tree was inferred using RAxML with a subset of sequences that were randomly subsampled (n = 505) and multiple-sequence aligned with MAFFT with the algorithm FFT-NS-I (Stamatakis, 2006; Nakamura et al., 2018). The phylogenetic tree was rooted with the reference sequence (accession number: MN908947) (Stamatakis, 2014; Rozewicki et al., 2019).
2.6. Quantification of circulating variants in wastewater using digital droplet PCR
Droplet digital PCR (ddPCR) was used to quantify circulating variants in wastewater using two assays designed by BioRad to detect the presence of the alpha (B.1.1.7) variant and the delta (B.1.617.2) variant. The B.1.1.7 lineage assay targeted the A23063T single nucleotide variant (SNV) in the spike gene (N501Y, FAM probe, Table S3). Similarly, the B.1.617.2 lineage assay targeted the T22917G SNV in the spike gene (L452R, FAM probe, Table S3). ddPCR assays were set up in 22 μl volumes using the One-Step RT-ddPCR Advanced kit for Probes (BioRad, USA) according to the manufacturer's guidelines with 1.1 μl 20× multiplex assay, water and 5 μl of sample RNA. To generate droplets, 20 μl of reaction mix was added to a droplet generation cartridge along with 70 μl of droplet generation oil. Droplets were then generated using the BioRad QX200 droplet generator (BioRad, USA) and 40 μl volumes of droplet partitioned PCR reaction mix was transferred to 96-well plates. The thermocycling conditions are listed in Table S3. The reaction mix was then held at 12 °C for 15 min before holding at 4 °C. From each reaction, droplets were counted and assigned as positive or negative using the QX200 reader and QX software (BioRad, US, Table S1). The QX software calculated the concentration of target amplicon in the sample based on the proportion of positive droplets assuming RNA partitioned into the droplets following a Poisson distribution. SARS-CoV-2 Alpha or Delta variant RNA was used as positive controls for all reactions to determine the threshold between positive and negative droplets (Fig. S1).
2.7. Data analysis
The concentrations (per 100 ml) of the SARS-CoV-2 N1 marker and both the Alpha and Delta variants and the volume flowing through the Ringsend WWTP was used to determine the loading per day [concentration (/100 ml) x flow (m3) x (10,000)]. For graphing purposes, samples in which the Alpha and Delta variant could not be detected, gc/100 ml and gc/day were calculated by multiplying the lowest Alpha or Delta concentration observed by 0.25. This value of 0.25 times the lowest concentration was used to account for the amount of sewage analysed per ddPCR reaction which ranged from approximately 2.5 to 10 μl (Table S1).
COVID-19 is a notifiable disease in Ireland under Infectious Diseases Regulations (Regulations is the Infectious Diseases (Amendment) Regulations 2020 (S.I. No. 53 of 2020). Notifications are registered on the Computerised Infectious Disease Reporting System (CIDR). Data on confirmed COVID-19 cases notified to the HSE Health Protection Surveillance Centre were obtained from covid-19.geohive.ie. The time series of COVID-19 case counts and SARS-CoV-2 wastewater viral concentrations and daily loadings were smoothed using generalised additive models (GAMs) (Wood, 2011). These smoothed time series were then log10 transformed, and a series of first order differences were calculated for each of them (i.e. time series of changes from one time point to the next). Finally, the time series of first order differences of COVID-19 cases was related to those for viral concentration and load using Spearman rank correlations (ρ). This analysis was conducted using R version 4.0.3 and GAMs were fit using the mgcv package (R Core Team, 2020; Wood, 2011). Additionally, a grid search was employed to consider a range of lead and lag times between SARS-CoV-2 wastewater and case-based data ranging from −6 to 6 weeks (LaValle et al., 2004). For statistical analyses, wastewater samples with levels of SARS-CoV-2 N1 below the detection limit of the assay were given the concentration of 1.25 gc/reaction, one quarter of the quantification limit of this assay.
3. Results
3.1. Wastewater surveillance of SARS-CoV-2 in Dublin
Composite wastewater samples were collected from the Ringsend WWTP in Dublin between June 2020 and August 2021 in order to monitor the prevalence of infection in the region. This period encompassed fully the second and third waves of the COVID-19 pandemic in Ireland. During these waves in Dublin, new daily cases peaked at 396 on the 17th of October 2020 and 3647 on the 3rd of January 2021 respectively. The results of monitoring SARS-CoV-2 RNA by RT-qPCR in Ringsend composite influent demonstrated that both N1 gene concentrations and daily loadings from the greater Dublin area mirrored trends in the reported case-based data (Fig. 1A, B). Furthermore, peak SARS-CoV-2 N1 gene daily loadings were observed in early November 2020 during the second wave and in January 2021 during the third wave (Fig. 1A, B). Following this third wave peak, SARS-CoV-2 levels decreased and remained relatively stable in Ringsend wastewater for the remainder of the study period, although sporadic increases were observed in March and May that were not reflected in new case-based data. The time series of first-order differences in COVID-19 cases in Dublin (preceding sample collection) were correlated with the time-series of first order differences in SARS-CoV-2 N1 daily loadings (Spearman's ρ = 0.50, p < 0.001) and concentrations (Spearman's ρ = 0.49, p < 0.001, Fig. S2). Furthermore, to determine if there was a lead or lag time between SARS-CoV-2 clinical cases and wastewater signals we used a grid search to consider a range of lag times ranging from −6 to +6 weeks and found the strongest correspondence between wastewater viral loading and cases when there was no lag (lag = 0).
Fig. 1.
Daily SARS-CoV-2 loading in wastewater and number of SARS-CoV-2 positive cases. A) The new daily cases in Dublin (incident cases) are represented by gray bars. The 5-day average of new cases preceding wastewater sampling are indicated as black dots on the graph. The Ringsend geo-coded cases for the NWSP are indicated by the red line. B) The daily loadings of the SARS-CoV-2 N1 gene (gc/day) through the Ringsend wastewater treatment plant are illustrated as black dots. The black line depicts the generalised additive model smoothing of the SARS-CoV-2 N1 daily loading data.
3.2. Assessment of circulating lineages
During the COVID-19 pandemic, the UCD National Virus Reference Laboratory has sequenced representative SARS-CoV-2 positive samples from the pandemic in Ireland and deposited them in GISAID. A total of 9187 sequences from the 82,000 new cases detected during the sampling period in Dublin (~11% sequenced) were further considered to analyse the distribution of lineages during the considered period of the pandemic. A total of 115 different PANGOLIN lineages were identified during the sampling period, however, as many of the lineages were related, we summarized different lineages according to their super lineages (e.g. B.1.177.*, B.1.258.*, AZ.*, etc.) including the VOCs and interest (e.g. Alpha, Delta, etc.).
The phylogenetic relationships among the sequenced lineages were explored with a phylogenetic tree considering a subsample (n = 505) of all available sequences (n = 9187) (Fig. 2A). The Alpha variant showed clear distinctions to other sequences at both phylogenetic and temporal levels (Fig. 2B). Furthermore, the temporal distribution of super lineages characterized each of the epidemiological waves, with the second wave characterized by a surge of cases represented by genome sequences segregating in lineages B.1.177.* and B.1.258.* (Fig. 3 B). On the other hand, the third wave was associated with the introduction of the Alpha variant and a rapid increase in the numbers of clinical cases (Fig. 1A) associated with this variant (Fig. 3B). A fourth wave has been characterized by the replacement of the Alpha variant by lineages derived from the Delta variant. It is noteworthy that during the third wave, other variants were identified in Ireland but only the Delta variant displaced the Alpha variant as the prevalent lineage during the fourth wave.
Fig. 2.
Phylogenetic and temporal relationships among SARS-CoV-2 genome sequences. A) Phylogenetic tree of SARS-CoV-2 complete genome sequences from clinical samples in Dublin (n = 505) rooted with the reference genome. The horizontal axis corresponds to the length of the branches and represents the number of nucleotide mutations per site. Tips are coloured according to the corresponding PANGOLIN lineage grouped by variant/super lineage on the right of the fig. B) Scatterplot reflecting the phylogenetic position of the tree tips against the collection date of the respective samples in the horizontal axis.
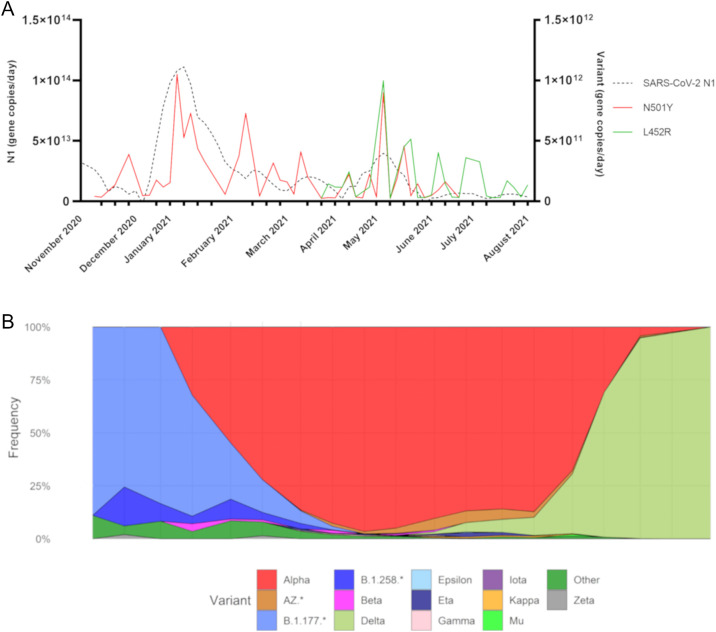
Fig. 3.
Circulating lineages and Quantification of SARS-CoV-2 Alpha and Delta Variants in Ringsend Wastewater. A) The date of wastewater sample collection is indicated on the X-axis. The left Y-axis indicates the daily loading of the SARS-CoV-2 N1 gene. The right Y-axis illustrates the daily loadings of both variants. The dashed black line is the smoothed curve depicting fluctuations in SARS-CoV-2 N1. The red and green lines depict the loadings of the Alpha (N501Y) and Delta (L452R) variants in the Ringsend wastewater treatment plant. B) Over the same period, percentages of Dublin cases per super-lineage during the sampling period are shown. Sequences are grouped biweekly, and the vertical axis represents the percentage of cases in each super lineage considering all available sequence data (n = 9187).
3.3. Identifying circulating VOCs in Dublin by monitoring wastewater
To complement the variant data obtained from genome sequencing of SARS-CoV-2 positive clinical samples, ddPCR was used to determine if the Alpha variant signal in Ringsend influent increased during the third wave as was observed from the sequencing of clinical samples. The earliest date that the N501Y SNV, A23063T, used as a proxy for the Alpha variant, could be detected in Ringsend influent was the 23rd of November 2020 (Fig. 3A), whereas the first genome sequence of an Alpha variant from a clinical sample collected on the 17th of December was reported on the 24th of December 2020. It should be noted that retrospective allelic discrimination qPCR analysis detected the Alpha variant in clinical samples in Ireland at the end of October. Peak N501Y concentrations in Ringsend influent were observed on the 6th of January which coincides with the peaks observed in case-based data and SARS-CoV-2 N1 concentrations in wastewater during the third wave of infection.
ddPCR was also employed to identify the earliest date that the Delta variant could be detected in wastewater. The first date that the L452R SNV, used as a proxy for the Delta variant, was detected in Ringsend influent was the 7th of April 2021, which coincided with the first complete genome for the variant collected on the same date, four months after its first identification in the UK (source: https://www.gisaid.org/hcov19-variants/). During the sampling period, the highest concentrations of L452R were observed in May and June. The N501Y signal could still be detected in wastewater until June and the L452R peaks actually coincided with increased concentrations of N501Y and the SARS-CoV-2 N1 gene. L452R could be detected until the end of the sampling period which represented the beginning of Ireland's fourth wave of SARS-CoV-2 infections.
4. Discussion
This work details the application of wastewater surveillance coupled with the genome sequencing of clinical samples to better understand the dissemination of SARS-CoV-2 and identify the dominant lineages circulating in the greater Dublin area. SARS-CoV-2 N1 RNA signals from Ringsend wastewater followed a strikingly similar trend to the reported new daily COVID-19 cases in Dublin, particularly during the second and third infection waves. These results are further supported by the observed correlations between SARS-CoV-2 N1 RNA concentrations and daily loadings and case numbers in Dublin.
These results demonstrate that monitoring fluctuations of SARS-CoV-2 RNA in the Ringsend WWTP can provide insight into the spread of infection in the greater Dublin area. Previous studies have indicated that increasing levels of SARS-CoV-2 RNA in wastewater have been predictive of an upcoming increase in new clinical cases (Peccia et al., 2020; Randazzo et al., 2020). For instance, Peccia et al. identified increasing levels of SARS-CoV-2 RNA to precede clinical cases by 6–8 days. This relationship was not observed during the course of our study, which is likely a result of a number of factors including sewage system residence time and lag time between symptom onset, testing and result confirmation and notification (Krivoňáková et al., 2021; Weidhaas et al., 2021). Such factors contribute to the variability in lead and lag times that have been reported between clinical case and wastewater data, which has been highlighted in a recent review by Kumar et al. (Kumar et al., 2022). However, during the third wave of infections peak daily loadings of SARS-CoV-2 RNA were observed on the 3rd of January which also had the highest new daily case numbers in Dublin. The predictive power of wastewater monitoring for SARS-CoV-2 is greater when community testing is limited (Sharif et al., 2021). As such the overlap between peak wastewater and case-based data indicates the rapidness of the increase in infections during this wave in Ireland which has high clinical testing capacity. It should also be noted that as wastewater samples were not collected daily any differences between wastewater and case data may be a result of sampling frequencies differing. Following the third wave of infections, SARS-CoV-2 N1 concentrations and daily loadings remained relatively stable and low for the remainder of the study period which was also observed for case-based data. This is likely a result of Ireland instituting mitigation measures limiting social gatherings, travel and work during this period. Furthermore, Ireland's vaccination programme began in early 2021 and by the end of this study period approximately 56% of the population had been fully vaccinated. Interestingly, sporadic increases in SARS-CoV-2 concentrations and loadings were observed in March and May where no concomitant increase in case numbers was observed. This may be a result of increased transmission occurring in the greater Dublin area which was not detected by clinical testing.
Although the presence of the SNV A23063T corresponding to S:N501Y was largely associated with the Alpha variant, during the period spanning the third epidemic wave multiple lineages independently acquired this SNV such as variants Beta, Gamma and Mu (source: https://outbreak.info/compare-lineages). Analogously, the SNV T22917G (S:L452R) associated with the Delta variant was related to lineages S and Epsilon variants. Nevertheless, the sequencing of samples during this period showed the low prevalence of these variants in the region. Therefore, such results highlight the synergy between the wastewater analysis and the whole-genome sequencing of samples from the national surveillance efforts as complementary for the proper interpretation of the observations.
A number of RT-qPCR assays have been developed to identify and quantify SARS-CoV-2 variants in wastewater (Yaniv et al., 2021; Johnson et al., 2022). However, circulating SARS-CoV-2 variant targets in many cases will be present in concentrations too low to be quantified using traditional RT-qPCR. As such, we used dPCR to quantify the N501Y and L452R SNVs in wastewater samples as proxies for the presence of the Alpha and Delta variants respectively (Borchardt et al., 2021; Ho et al., 2022; Lou et al., 2022).
Using this method, the Alpha variant was identified in Ringsend wastewater samples in November three weeks prior to its genome identification from clinical samples in early December and two months after its first identification in the UK, Ireland's only bordering neighbour, in September 2020 (Rambaut et al., 2020b). The Delta variant on the other hand was first identified in wastewater and clinical samples on the same day. These data contrast with Heijnen et al. who found the Alpha variant in Utrecht wastewater two weeks after its first clinical identification (Heijnen et al., 2021). This observation may be a result of sequence capacity, which is supported by the retrospective identification of the Alpha variant, using RT-qPCR, in a clinical sample from Dublin predating the wastewater positive sample.
Furthermore, during the third infection wave, increased SARS-CoV-2 N1 levels in wastewater coincided with increased Alpha variant levels indicating that the introduction of this variant coupled with relaxed restrictions in Ireland in December 2020 contributed to increased transmission of the virus in Dublin. Interestingly, the sporadic increases in the SARS-CoV-2 N1 gene observed in wastewater were associated with increases in both the Alpha and Delta variants in May 2021. This suggests that this increase in SARS-CoV-2 N1 resulted from events when lockdown ended and restrictions were being eased, leading to increased transmission of all circulating variants in the greater Dublin area rather than the introduction of a single variant with increased transmission i.e., the Delta variant.
To conclude, we have demonstrated that SARS-CoV-2 wastewater monitoring data from a single large WWTP in Dublin reflected case data in the greater Dublin area. Moreover, the surveillance of VOCs in this WWTP reflected the results of clinical sample sequencing and also preceded, further demonstrating the potential utility of this approach to SARS-CoV-2 surveillance.
Funding
This work was part funded by the European Regional Development Fund through the Ireland Wales Cooperation programme (Acclimatize), by Science Foundation Ireland (20-CoV-0159) and by the Health Service Executive.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Editor: Damià Barceló
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.scitotenv.2022.155828.
Appendix A. Supplementary data
Supplementary material
References
- Ahmed W., Angel N., Edson J., Bibby K., Bivins A., O'Brien J.W., Choi P.M., Kitajima M., Simpson S.L., Li J., Tscharke B., Verhagen R., Smith W.J.M., Zaugg J., Dierens L., Hugenholtz P., Thomas K.V., Mueller J.F. First confirmed detection of sars-cov-2 in untreated wastewater in Australia: a proof of concept for the wastewater surveillance of covid-19 in the community. Sci. Total Environ. 2020;728 doi: 10.1016/j.scitotenv.2020.138764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baum A., Fulton Benjamin O., Wloga E., Copin R., Pascal Kristen E., Russo V., Giordano S., Lanza K., Negron N., Ni M., Wei Y., Atwal Gurinder S., Murphy Andrew J., Stahl N., Yancopoulos George D., Kyratsous Christos A. Antibody cocktail to sars-cov-2 spike protein prevents rapid mutational escape seen with individual antibodies. Science. 2020;369(6506):1014–1018. doi: 10.1126/science.abd0831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borchardt M.A., Boehm A.B., Salit M., Spencer S.K., Wigginton K.R., Noble R.T. The environmental microbiology minimum information (emmi) guidelines: qpcr and dpcr quality and reporting for environmental microbiology. Environ. Sci. Technol. 2021;55(15):10210–10223. doi: 10.1021/acs.est.1c01767. [DOI] [PubMed] [Google Scholar]
- CDC 2019-novel coronavirus (2019-ncov) real-time rrt-pcr panel primers and probes. 2020. https://www.cdc.gov/coronavirus/2019-ncov/downloads/rt-pcr-panel-primer-probes.pdf Available from. [Accessed 04.15.21]
- Chen Y., Chen L., Deng Q., Zhang G., Wu K., Ni L., Yang Y., Liu B., Wang W., Wei C., Yang J., Ye G., Cheng Z. The presence of sars-cov-2 rna in the feces of covid-19 patients. J. Med. Virol. 2020;92(7):833–840. doi: 10.1002/jmv.25825. [DOI] [PubMed] [Google Scholar]
- Team R.C. R foundation for statistical computing; 2020. R: a language and environment for statistical computing.http://www.r-project.org/index.html Available from. [Google Scholar]
- Feng S., Roguet A., McClary-Gutierrez J.S., Newton R.J., Kloczko N., Meiman J.G., McLellan S.L. Evaluation of sampling, analysis, and normalization methods for sars-cov-2 concentrations in wastewater to assess covid-19 burdens in Wisconsin communities. ACS ES&T Water. 2021 doi: 10.1021/acsestwater.1c00160. [DOI] [Google Scholar]
- Freed N.E., Vlková M., Faisal M.B., Silander O.K. Rapid and inexpensive whole-genome sequencing of sars-cov-2 using 1200 bp tiled amplicons and oxford nanopore rapid barcoding. Biol. Methods Protoc. 2020;5(1) doi: 10.1093/biomethods/bpaa014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Funk T., Pharris A., Spiteri G., Bundle N., Melidou A., Carr M., Gonzalez G., Garcia-Leon A., Crispie F., O’Connor L., Murphy N., Mossong J., Vergison A., Wienecke-Baldacchino A.K., Abdelrahman T., Riccardo F., Stefanelli P., Martino A.Di, Bella A., Presti A.Lo, Casaca P., Moreno J., Borges V., Isidro J., Ferreira R., Gomes J.P., Dotsenko L., Suija H., Epstein J., Sadikova O., Sepp H., Ikonen N., Savolainen-Kopra C., Blomqvist S., Möttönen T., Helve O., Gomes-Dias J., Adlhoch C., o.b.o.C.s. groups Characteristics of sars-cov-2 variants of concern b.1.1.7, b.1.351 or p.1: data from seven eu/eea countries, weeks 38/2020 to 10/2021. Eurosurveillance. 2021;26(16) doi: 10.2807/1560-7917.ES.2021.26.16.2100348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greaney A.J., Starr T.N., Gilchuk P., Zost S.J., Binshtein E., Loes A.N., Hilton S.K., Huddleston J., Eguia R., Crawford K.H.D., Dingens A.S., Nargi R.S., Sutton R.E., Suryadevara N., Rothlauf P.W., Liu Z., Whelan S.P.J., Carnahan R.H., Crowe J.E., Bloom J.D. Complete mapping of mutations to the sars-cov-2 spike receptor-binding domain that escape antibody recognition. Cell Host Microbe. 2021;29(1):44–57.e49. doi: 10.1016/j.chom.2020.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han H., Luo Q., Mo F., Long L., Zheng W. Sars-cov-2 rna more readily detected in induced sputum than in throat swabs of convalescent covid-19 patients. Lancet Infect. Dis. 2020;20(6):655–656. doi: 10.1016/S1473-3099(20)30174-2. 110.1016/S1473-3099(20)30174-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harvey W.T., Carabelli A.M., Jackson B., Gupta R.K., Thomson E.C., Harrison E.M., Ludden C., Reeve R., Rambaut A., Peacock S.J., Robertson D.L., C.-G.U. Consortium Sars-cov-2 variants, spike mutations and immune escape. Nat. Rev. Microbiol. 2021;19(7):409–424. doi: 10.1038/s41579-021-00573-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hata A., Honda R. Potential sensitivity of wastewater monitoring for sars-cov-2: comparison with norovirus cases. Environ. Sci. Technol. 2020;54(11):6451–6452. doi: 10.1021/acs.est.0c02271. [DOI] [PubMed] [Google Scholar]
- Heijnen L., Elsinga G., de Graaf M., Molenkamp R., Koopmans M.P.G., Medema G. Droplet digital rt-pcr to detect sars-cov-2 signature mutations of variants of concern in wastewater. Sci. Total Environ. 2021;799 doi: 10.1016/j.scitotenv.2021.149456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ho J., Stange C., Suhrborg R., Wurzbacher C., Drewes J.E., Tiehm A. Sars-cov-2 wastewater surveillance in Germany: long-term rt-digital droplet pcr monitoring, suitability of primer/probe combinations and biomarker stability. Water Res. 2022;210 doi: 10.1016/j.watres.2021.117977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson R., Sharma J.R., Ramharack P., Mangwana N., Kinnear C., Viraragavan A., Glanzmann B., Louw J., Abdelatif N., Reddy T., Surujlal-Naicker S., Nkambule S., Mahlangeni N., Webster C., Mdhluli M., Gray G., Mathee A., Preiser W., Muller C., Street R. Tracking the circulating sars-cov-2 variant of concern in South Africa using wastewater-based epidemiology. Sci. Rep. 2022;12(1):1182. doi: 10.1038/s41598-022-05110-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krivoňáková N., Šoltýsová A., Tamáš M., Takáč Z., Krahulec J., Ficek A., Gál M., Gall M., Fehér M., Krivjanská A., Horáková I., Belišová N., Bímová P., Škulcová A.B., Mackuľak T. Mathematical modeling based on rt-qpcr analysis of sars-cov-2 in wastewater as a tool for epidemiology. Sci. Rep. 2021;11(1):19456. doi: 10.1038/s41598-021-98653-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar M., Jiang G., Kumar Thakur A., Chatterjee S., Bhattacharya T., Mohapatra S., Chaminda T., Kumar Tyagi V., Vithanage M., Bhattacharya P., Nghiem L.D., Sarkar D., Sonne C., Mahlknecht J. Lead time of early warning by wastewater surveillance for covid-19: geographical variations and impacting factors. Chem. Eng. J. 2022;441:135936. doi: 10.1016/j.cej.2022.135936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- La Rosa G., Iaconelli M., Mancini P., Bonanno Ferraro G., Veneri C., Bonadonna L., Lucentini L., Suffredini E. First detection of sars-cov-2 in untreated wastewaters in Italy. Sci. Total Environ. 2020;736 doi: 10.1016/j.scitotenv.2020.139652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lan J., Ge J., Yu J., Shan S., Zhou H., Fan S., Zhang Q., Shi X., Wang Q., Zhang L., Wang X. Structure of the sars-cov-2 spike receptor-binding domain bound to the ace2 receptor. Nature. 2020;581(7807):215–220. doi: 10.1038/s41586-020-2180-5. [DOI] [PubMed] [Google Scholar]
- LaTurner Z.W., Zong D.M., Kalvapalle P., Gamas K.R., Terwilliger A., Crosby T., Ali P., Avadhanula V., Santos H.H., Weesner K., Hopkins L., Piedra P.A., Maresso A.W., Stadler L.B. Evaluating recovery, cost, and throughput of different concentration methods for sars-cov-2 wastewater-based epidemiology. Water Res. 2021;197 doi: 10.1016/j.watres.2021.117043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LaValle S.M., Branicky M.S., Lindemann S.R. On the relationship between classical grid search and probabilistic roadmaps. Int. J. Robot. Res. 2004;23(7-8):673–692. doi: 10.1177/0278364904045481. Available from doi:10.1177/0278364904045481 [Accessed 2022/04/11] [DOI] [Google Scholar]
- Leal Filho W., Brandli L.L., Lange Salvia A., Rayman-Bacchus L., Platje J. Covid-19 and the un sustainable development goals: threat to solidarity or an opportunity? Sustainability. 2020;12(13) doi: 10.3390/su12135343. [DOI] [Google Scholar]
- Lou E.G., Sapoval N., McCall C., Bauhs L., Carlson-Stadler R., Kalvapalle P., Lai Y., Palmer K., Penn R., Rich W., Wolken M., Brown P., Ensor K.B., Hopkins L., Treangen T.J., Stadler L.B. Direct comparison of rt-ddpcr and targeted amplicon sequencing for sars-cov-2 mutation monitoring in wastewater. Sci. Total Environ. 2022;155059 doi: 10.1016/j.scitotenv.2022.155059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu X., W.L., Sakthivel S.K., Whitaker B., Murray J., Kamili S., et al. Us cdc real-time reverse transcription pcr panel for detection of severe acute respiratory syndrome coronavirus 2. Emerg. Infect. Dis. 2020;26(8):1654–1665. doi: 10.3201/eid2608.201246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Medema G., Heijnen L., Elsinga G., Italiaander R., Brouwer A. Presence of sars-coronavirus-2 rna in sewage and correlation with reported covid-19 prevalence in the early stage of the epidemic in the Netherlands. Environ. Sci. Technol. Lett. 2020;7(7):511–516. doi: 10.1021/acs.estlett.0c00357. [DOI] [PubMed] [Google Scholar]
- Motozono C., Toyoda M., Zahradnik J., Saito A., Nasser H., Tan T.S., Ngare I., Kimura I., Uriu K., Kosugi Y., Yue Y., Shimizu R., Ito J., Torii S., Yonekawa A., Shimono N., Nagasaki Y., Minami R., Toya T., Sekiya N., Fukuhara T., Matsuura Y., Schreiber G., Ikeda T., Nakagawa S., Ueno T., Sato K. Sars-cov-2 spike l452r variant evades cellular immunity and increases infectivity. Cell Host Microbe. 2021;29(7):1124–1136.e1111. doi: 10.1016/j.chom.2021.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakamura T., Yamada K.D., Tomii K., Katoh K. Parallelization of mafft for large-scale multiple sequence alignments. Bioinformatics. 2018;34(14):2490–2492. doi: 10.1093/bioinformatics/bty121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Toole Á., Scher E., Underwood A., Jackson B., Hill V., McCrone J.T., Colquhoun R., Ruis C., Abu-Dahab K., Taylor B., Yeats C., du Plessis L., Maloney D., Medd N., Attwood S.W., Aanensen D.M., Holmes E.C., Pybus O.G., Rambaut A. Assignment of epidemiological lineages in an emerging pandemic using the pangolin tool. Virus Evol. 2021;7(2) doi: 10.1093/ve/veab064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peccia J., Zulli A., Brackney D.E., Grubaugh N.D., Kaplan E.H., Casanovas-Massana A., Ko A.I., Malik A.A., Wang D., Wang M., Warren J.L., Weinberger D.M., Arnold W., Omer S.B. Measurement of sars-cov-2 rna in wastewater tracks community infection dynamics. Nat. Biotechnol. 2020;38(10):1164–1167. doi: 10.1038/s41587-020-0684-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polack F.P., Thomas S.J., Kitchin N., Absalon J., Gurtman A., Lockhart S., Perez J.L., Marc G.Pérez, Moreira E.D., Zerbini C., Bailey R., Swanson K.A., Roychoudhury S., Koury K., Li P., Kalina W.V., Cooper D., Frenck R.W., Jr., Hammitt L.L., Türeci Ö., Nell H., Schaefer A., Ünal S., Tresnan D.B., Mather S., Dormitzer P.R., Şahin U., Jansen K.U., Gruber W.C., C.C.T. Group Safety and efficacy of the bnt162b2 mrna covid-19 vaccine. N. Engl. J. Med. 2020;383(27):2603–2615. doi: 10.1038/s41564-020-0770-510.1056/NEJMoa2034577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rambaut A., Holmes E.C., O’Toole Á., Hill V., McCrone J.T., Ruis C., du Plessis L., Pybus O.G. A dynamic nomenclature proposal for sars-cov-2 lineages to assist genomic epidemiology. Nat. Microbiol. 2020;5(11):1403–1407. doi: 10.1038/s41564-020-0770-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rambaut A., Loman N., Pybus O., Barclay W., Barrett J., Carabelli A., Connor T., Peacock T., Robertson D.L., Volz E., CoG-UK Preliminary genomic characterisation of an emergent sars-cov-2 lineage in the uk defined by a novel set of spike mutations. 2020. https://virological.org/t/preliminary-genomic-characterisation-of-an-emergent-sars-cov-2-lineage-in-the-uk-defined-by-a-novel-set-of-spike-mutations/563 Available from.
- Randazzo W., Truchado P., Cuevas-Ferrando E., Simón P., Allende A., Sánchez G. Sars-cov-2 rna in wastewater anticipated covid-19 occurrence in a low prevalence area. Water Res. 2020;181:115942. doi: 10.3389/fviro.2021.776998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rozewicki J., Li S., Amada K.M., Standley D.M., Katoh K. Mafft-dash: integrated protein sequence and structural alignment. Nucleic Acids Res. 2019;47(W1):W5–W10. doi: 10.1093/nar/gkz342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rutledge R.G., Côté C. Mathematics of quantitative kinetic pcr and the application of standard curves. Nucleic Acids Res. 2003;31(16) doi: 10.1093/nar/gng093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rutledge R.G., Stewart D. Critical evaluation of methods used to determine amplification efficiency refutes the exponential character of real-time pcr. BMC Mol. Biol. 2008;9:96. doi: 10.1186/1471-2199-9-96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shah S., Gwee S.X.W., Ng J.Q.X., Lau N., Koh J., Pang J. Wastewater surveillance to infer covid-19 transmission: a systematic review. Sci. Total Environ. 2022;804 doi: 10.1016/j.scitotenv.2021.150060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharif S., Ikram A., Khurshid A., Salman M., Mehmood N., Arshad Y., Ahmed J., Safdar R.M., Rehman L., Mujtaba G., Hussain J., Ali J., Angez M., Alam M.M., Akthar R., Wasif Malik M., Iqbal Baig M.Z., Suleman Rana M., Usman M., Qaisar Ali M., Ahad A., Badar N., Umair M., Tamim S., Ashraf A., Tahir F., Ali N. Detection of sars-cov-2 in wastewater using the existing environmental surveillance network: a potential supplementary system for monitoring covid-19 transmission. PloS one. 2021;16(6) doi: 10.1371/journal.pone.0249568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stamatakis A. Raxml-vi-hpc: maximum likelihood-based phylogenetic analyses with thousands of taxa and mixed models. Bioinformatics. 2006;22(21):2688–2690. doi: 10.1093/bioinformatics/btl446. [DOI] [PubMed] [Google Scholar]
- Stamatakis A. Raxml version 8: a tool for phylogenetic analysis and post-analysis of large phylogenies. Bioinformatics. 2014;30(9):1312–1313. doi: 10.1093/bioinformatics/btu033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tian F., Tong B., Sun L., Shi S., Zheng B., Wang Z., Dong X., Zheng P. N501y mutation of spike protein in sars-cov-2 strengthens its binding to receptor ace2. eLife. 2021;10 doi: 10.7554/eLife.69091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Twomey J.D., Luo S., Dean A.Q., Bozza W.P., Nalli A., Zhang B. Covid-19 update: the race to therapeutic development. Drug Resist. Updat. 2020;53 doi: 10.1016/j.drup.2020.100733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Viveros M.L., Azimi S., Pichon E., Roose-Amsaleg C., Bize A., Durandet F., Rocher V. Research Square; 2021. Wild Type and Variants of sars-cov-2 in Parisian Sewage: Dynamics in Raw Water and Fate in Wastewater Treatment Plants. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weidhaas J., Aanderud Z.T., Roper D.K., VanDerslice J., Gaddis E.B., Ostermiller J., Hoffman K., Jamal R., Heck P., Zhang Y., Torgersen K., Laan J.V., LaCross N. Correlation of sars-cov-2 rna in wastewater with covid-19 disease burden in sewersheds. Sci. Total Environ. 2021;775:145790. doi: 10.1016/j.scitotenv.2021.145790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wood S.N. Fast stable restricted maximum likelihood and marginal likelihood estimation of semiparametric generalized linear models. J. R. Stat. Soc. Series. B. Stat. Methodol. 2011;73(1):3–36. doi: 10.1111/j.1467-9868.2010.00749.x. [DOI] [Google Scholar]
- Yaniv K., Ozer E., Shagan M., Lakkakula S., Plotkin N., Bhandarkar N.S., Kushmaro A. Direct rt-qpcr assay for sars-cov-2 variants of concern (alpha, b.1.1.7 and beta, b.1.351) detection and quantification in wastewater. Environ. Res. 2021;201:111653. doi: 10.1016/j.envres.2021.111653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou P., Yang X.-L., Wang X.-G., Hu B., Zhang L., Zhang W., Si H.-R., Zhu Y., Li B., Huang C.-L., Chen H.-D., Chen J., Luo Y., Guo H., Jiang R.-D., Liu M.-Q., Chen Y., Shen X.-R., Wang X., Zheng X.-S., Zhao K., Chen Q.-J., Deng F., Liu L.-L., Yan B., Zhan F.-X., Wang Y.-Y., Xiao G.-F., Shi Z.-L. A pneumonia outbreak associated with a new coronavirus of probable bat origin. Nature. 2020;579(7798):270–273. doi: 10.1038/s41586-020-2012-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary material