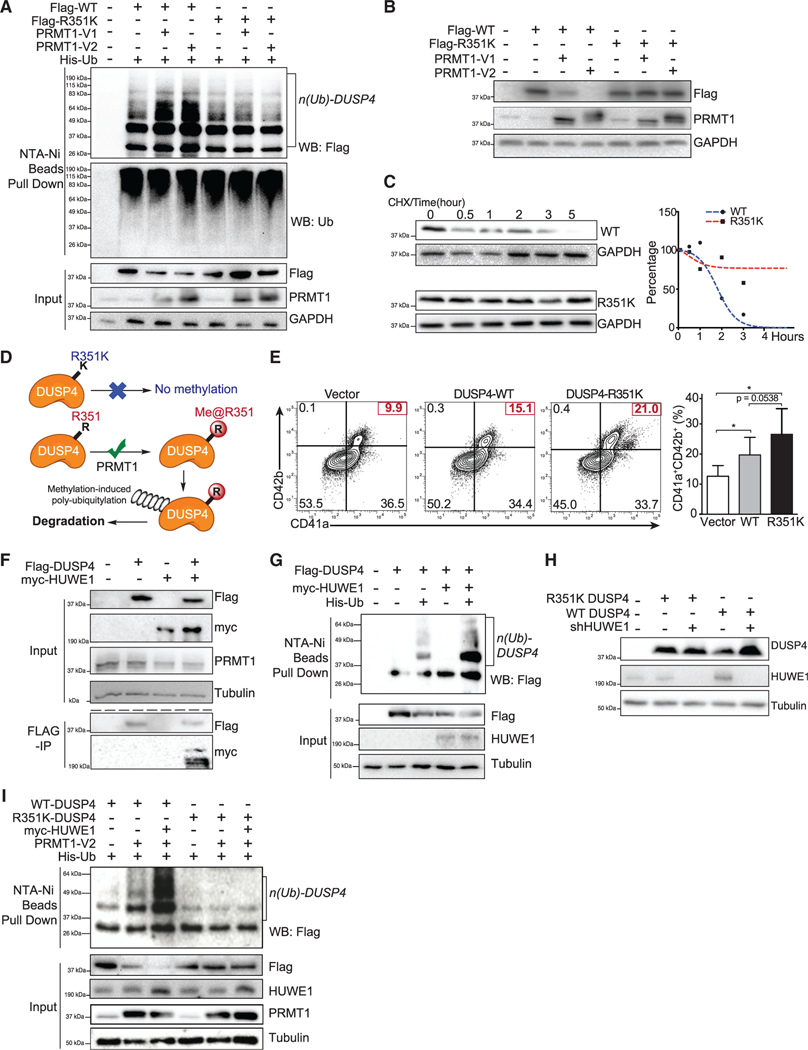
Figure 6. Polyubiquitylation and instability of DUSP4 promoted by PRMT1-involved R351 methylation.
For a Figure360 author presentation of this figure, see https://doi.org/10.1016/j.celrep.2021.109421.
(A) Polyubiquitylation of DUSP4, but not its R351K variant, is stimulated by PRMT1. 293T cells were transfected with DUSP4 (wild-type and R351K mutant), 6× His-tagged ubiquitin, and PRMT1 (V1 and V2 isoforms). Cells were treated with MG132 for 6 h prior to harvest. Fractions of pull-downs (upper panel) and inputs (bottom panel) were applied for western blotting (n = 3, representative western blots).
(B) PRMT1-dependent stability levels of wild-type and R351K DUSP4 were determined in 293T cells by western blotting (n = 3, representative western blots).
(C) Half-life time of wild-type and R351K DUSP4. Normalized protein stability curves are plotted in the right panel (n = 3, representative western blots).
(D) Mechanistic description of DUSP4 stability modulated by PRMT1-dependent R351 methylation. R351 methylation of DUSP4 by PRMT1 triggers its polyubiquitylation and thus its degradation; R351K mutation abolishes the methylation and thus suppresses polyubiquitylation and degradation (n = 3, representative western blots).
(E) Mk differentiation in the presence of wild-type and R351K DUSP4. Human CD34+ cells infected with lentiviruses expressing DUSP4s (wild-type or R351K mutant) were induced for Mk differentiation. Percentage of CD41a+CD42b+ cells was determined by FACS after 7 days. Representative plots and statistics are shown (n = 3, independent experiments). Data are shown as mean ± SD. Two-tailed paired t test, *p ≤ 0.05.
(F) Co-immunoprecipitation of HUWE1 and DUSP4. Flag-tagged DUSP4 was used to immunoprecipitate myc-tagged HUWE1 in co-transfected 293T cells (n = 3, representative western blots after immunoprecipitation).
(G) The protein ubiquitylation of DUSP4 is measured in 293T cells transfected with plasmids as shown on top of the gels (n = 3, representative western blots).
(H) Mutant and wild-type DUSP4 were expressed together with HUWE1 shRNA in 293T cells for western blotting with respective antibodies (n = 3, representative western blots).
(I) Protein ubiquitylation assays with wild-type and mutant DUSP4. DUSP4 wild-type protein and mutant protein were expressed in 293T cells transfected with or without the plasmid combination of HUWE1 and PRMT1 as indicated on the top of the gel for affinity purification with Ni-NTA beads (n = 3, representative western blots).