Abstract
BriLife®, a vector-based vaccine that utilizes the recombinant vesicular stomatitis virus (VSV) platform to express and present the spike antigen of SARS-CoV-2, is undergoing testing in a phase 2 clinical trial in Israel. A nonclinical repeated-dose (GLP) toxicity study in New Zealand white rabbits was performed to evaluate the potential toxicity, local tolerance, immunogenicity and biodistribution of the vaccine. rVSV-ΔG-SARS-CoV-2-S (or vehicle) was administered intramuscularly to two groups of animals (106, 107 PFU/animal, n = 10/sex/group) on three occasions, at 2-week intervals, followed by a 3-week recovery period. Systemic clinical signs, local reactions, body weight, body temperature, food consumption, ophthalmology, urinalysis, clinical pathology, C-reactive protein, viremia and antibody levels were monitored. Gross pathology was performed, followed by organs/tissues collection for biodistribution and histopathological evaluation. Treatment-related changes were restricted to multifocal minimal myofiber necrosis at the injection sites, and increased lymphocytic cellularity in the iliac and mesenteric lymph nodes and in the spleen. These changes were considered related to the inflammatory reaction elicited, and correlated with a trend for recovery. Detection of rVSV-ΔG-SARS-CoV-2-S vaccine RNA was noted in the regional iliac lymph node in animals assigned to the high-dose group, at both termination time points. A significant increase in binding and neutralizing antibody titers was observed following vaccination at both vaccine doses. In view of the findings, it was concluded that the rVSV-ΔG-SARS-CoV-2-S vaccine is safe. These results supported the initiation of clinical trials.
Supplementary Information
The online version contains supplementary material available at 10.1007/s00204-022-03302-5.
Keywords: Safety, Nonclinical, Vaccine, COVID-19, Neutralizing antibodies
Introduction
Coronavirus disease (COVID-19) is an infectious disease caused by the SARS-CoV-2 virus. The first case was reported to occur in Wuhan, China, in 2019, and subsequently the disease has spread rapidly globally to cause a global pandemic.
The urgent need for an efficacious vaccine for SARS-CoV-2 led to unprecedented innovations in vaccine development worldwide including protein subunit vaccines, both RNA and DNA-based vaccines, inactivated viruses, and viral vector-based vaccines, among others. Pfizer-BioNTech’s vaccine COMIRNATY® was the first COVID-19 vaccine to receive a conditional marketing authorization in the EU (21 December 2020) for prevention of COVID-19 in individuals 16 years of age and older: since then, 35 vaccines have received regulatory approval in at least one territory and a total of 10.5 × 109 vaccine doses have been administered worldwide (WHO Coronavirus Dashboard. Available online https://covid19.who.int/).
BriLife® is a vector-based vaccine that utilizes the recombinant vesicular stomatitis virus (VSV) platform to express and present the spike antigen of SARS-CoV-2 (Yahalom-Ronen et al. 2020). This vaccine, developed by the Israel Institute for Biological Research (IIBR), is currently undergoing clinical testing (Phase 2) in Israel. Wild-type (WT) VSV infects livestock and is not virulent to humans. WT-VSV infection is mediated by the viral envelope glycoprotein G protein that is involved in receptor recognition at the host cell surface, allowing membrane fusion and endocytosis. During development of BriLife®, the G gene was deleted from the viral genome and the S gene of SARS-CoV-2 was cloned into the VSV-ΔG backbone for expression on the virus surface and in infected cells. This approach is similar to that used by Merck Sharp & Dohme B.V. for ERVEBO®, a vaccine against Ebola virus disease (Suder et al. 2018).
Administration of single dose of rVSV-ΔG-SARS-CoV-2-S vaccine to golden Syrian hamsters, a well-established in vivo model for COVID-19, was well tolerated and resulted in a rapid and potent induction of SARS-CoV-2 neutralizing antibodies, and following SARS-CoV-2 challenge, vaccinated hamsters exhibited abrogation of body weight loss, alleviation of extensive tissue damage and reduced viral loads in the lungs and nasal turbinates (Yahalom-Ronen et al. 2020). We recently reported the results of preliminary nonclinical safety, immunogenicity and efficacy (potency) studies, conducted in four species: mouse, hamster, rabbit and pig (Madar-Balakirski et al. 2022). In these studies, rVSV-ΔG-SARS-CoV-2-S vaccine was found to be safe and immunogenic, with no treatment-related mortalities, or any noticeable systemic or local clinical signs. Hematology and biochemistry parameters were unremarkable as comparted to unvaccinated controls, no evidence for neurovirulence was found in two sensitive animal models (C57BL/6 immune-competent mice and type I interferon knock-out mice) and no adverse histopathological findings were observed. The vaccine induced neutralizing antibodies and cellular immune response. Increased lymphocytic cellularity in the spleen germinal centers and regional lymph node observed in rabbits was in line with expectations following immune stimulation with an immunogenic vaccine.
To further support the development of the rVSV-ΔG-SARS-CoV-2-S vaccine, a nonclinical repeated-dose toxicity study in New Zealand white (NZW) rabbits was performed in compliance with Good Laboratory Practice (GLP) principles. The study design was based on the principles described in the WHO Guideline of Nonclinical Evaluation of Vaccines-Annex 1 (Available on line https://www.who.int/publications/m/item/annex1 nonclinical.p31-63) and the EMA Guideline on quality, nonclinical and clinical aspects of live recombinant viral vectored vaccines (Available on line https://www.ema.europa.eu/en/quality-non-clinical-clinical-aspects-live-recombinant-viral-vectored-vaccines). The objective of the study was to assess the potential toxicity, local tolerance, immunogenicity and biodistribution of rVSV-ΔG-SARS-CoV-2-S vaccine, following three repeated vaccination sessions, by intramuscular (i.m.) injection at 2-week intervals (treatment start, 14- and 28-day post-first dosing), to male and female NZW rabbits with a 3-week recovery period. This study was part of the nonclinical package that supported the clinical testing of rVSV-ΔG-SARS-CoV-2-S vaccine (NCT04608305).
Materials and methods
Animals
Specific pathogen-free (SPF) male and female NZW (HsdOkd:NZW) rabbits (Envigo RMS (Israel)) were 3–4 months of age at study initiation. Weight at the time of treatment initiation ranged between 2.2 and 3.0 kg for males and 2.0 and 3.3 kg for females. Animals were given a unique identification ear number (tattooed by the breeder). Once received at the testing facility (Envigo CRS Israel Limited, Ness-Ziona, Israel), the animals were randomly assigned to the various test groups according to a random table list. The animal’s number appeared on a cage card visible on the front of each cage. Selection of animals for the various time points of termination (Main Phase or Recovery Phase) was performed arbitrarily upon cage number order (ascending order). The health status of the animals used in the study was examined on arrival. Only animals in good health were acclimatized to laboratory conditions for 11 days prior to study initiation. Rabbits were housed individually in plastic cages, arranged in batteries. Cages were fitted with perforated plastic floors above under-trays. Enrichment of the cage environment, in part or in whole, was provided in the form of supplemental hay and rabbit scenting bricks. Animals were provided a commercial rabbits’ diet, approximately 100 g/rabbit/day and allowed free access to drinking water. The water was supplied to each cage via polyethylene bottles with stainless steel sipper tubes. The water was chlorinated and acidified. The room temperature was 17–23 °C with a relative humidity (RH) between 30 and 70%. A 12-h light/dark cycle was provided via an automatic timer.
Ethics statement
This study was performed following an application-form review by the Israeli National Council for Animal Experimentation and after receiving approval (No. IL-20–6-249) that the study complies with the rules and regulations set forth.
Test and control items preparation
All the test materials [rVSV-ΔG-SARS-CoV-2-S and formulations buffer (4% trihalose, 150 mM NaCl, 2.5 mg/ml human serum albumin, 20 mM Tris)] were supplied as ready-to-use materials in frozen condition. Before each dosing session, one bottle of each material was thawed at room temperature (~ 30 min) and kept at 2-8ºC until delivery to the animal house. To maintain homogeneity during thawing, the vial was gently shaken. The test item was administered at ambient conditions.
Cells
Vero E6 cells (ATCC CRL-1586™) were grown in DMEM containing 10% fetal bovine serum (FBS), MEM nonessential amino acids (NEAA), 2 mM l-glutamine, 100 units/mL penicillin, 0.1 mg/mL streptomycin, and 12.5 units/mL nystatin (P/S/N). Calu3 cells (ATCC HTB-55) were grown in RPMI supplemented with 10% FBS, NEAA, 2 mM L-glutamine, P/S/N, and 1% Na-pyruvate. All reagents were from Biological Industries, Beit-Haemek, Israel. Cells were cultured at 37 °C, 5% CO2 with 95% humidity.
Procedures
The study included three groups of n = 20 animals (10 males and 10 females) per group (Table S1). The test item, rVSV-ΔG-SARS-CoV-2-S vaccine, was administered to two test groups, at two dose levels of 106 and 107 PFU/animal (low- and high-dose groups, respectively). The high-dose level represents the intended highest dose (in absolute terms) used in the first clinical trial and the low-dose level is tenfold lower. An additional group administered with the vehicle/formulation’s buffer served as a control group. The dosing schedule was intended to cover the number of injections foreseen in humans plus one extra. On each day of dosing, the first group subjected to dosing was the control group, followed by the low-dose group and then the high-dose group. The rVSV-ΔG-SARS-CoV-2-S vaccine and formulation buffer were administered i.m., into the right and left quadriceps (thigh) muscles in a designated injection site which was marked by four dots (tattoo). Injections were performed using a 2.5 ml syringe attached to a 25G hypodermic needle. A total volume of 1 ml/rabbit per dosing day was divided into the right and left injection sites: about 0.5 ml/site. The first i.m. administration was to the distal area of the right and left quadriceps muscle, while the second and third administrations were to the mid and proximal area of the right and left quadriceps muscle, respectively. Each animal was subjected to 3 i.m. administrations with an interval of 2 weeks between each treatment administration.
In-life examinations
The study outline and in-life examination are summarized in Table S2. Animals were observed (i.e., morbidity and mortality checks and/or cage-side observations) once to twice daily throughout the study period. Detailed clinical sign examinations were performed once weekly. Examinations of local reactions at injection site/s and scoring (according to the Draize scoring system) were performed prior to each dosing session, on each day of dosing 4–6 h after injection, daily for 3 days post-each dosing session, and once weekly on a non-dosing week or recovery period.
Body weight was determined prior to the first dosing session, on each day of the dosing session (pre-dosing), 3 days post each dosing session and once weekly on a non-dosing week or recovery period. Food consumption was measured once during the acclimation period, on the day of each dosing session, 3 days post each dosing session and once weekly on every other non-dosing week. Rectal temperature was measured twice during acclimation, on each day of dosing 4–6 h after injection, daily for 3 days post each dosing session. All animals were examined by indirect ophthalmoscopy during the last week prior to each respective necropsy time point.
Urine samples for urinalysis were collected from all animals on the day of each scheduled termination. The following parameters were determined by the use of a commercial test kit (Urit 10 V Urinalysis reagent strips): glucose, ketone, ph, leukocyte, blood, specific gravity (density), nitrite, bilirubin, urobilinogen and protein. Urine appearance (e.g., clear or opaque sample) and color were recorded.
Blood sampling for clinical pathology, CRP, antibody and viremia levels
Hematology, biochemistry and coagulation parameters were determined for all animals, at the following time points: once prior to the first dosing, 3 days post the first dosing and on the day of necropsy. Following completion of blood collection, all samples (whole blood, serum and plasma samples) were kept at 2-8ºC until transported to American Medical Laboratories (Israel) Ltd. for analysis.
For hematology parameters, blood was collected into potassium EDTA tubes and analyzed using the ADVIA 120 Hematology Analyzer. For clinical chemistry parameters, blood was collected into non-coated tubes and analyzed using ROCHE-COBAS 6000 C501 Analyzer. For coagulation parameters, blood was collected into citrate tubes and analyzed using Sysmex CA-1500.
Blood samples for determination of C-reactive protein (CRP) concentration were collected from all animals assigned to the recovery phase (5 males and 5 females per group), at the following time points: once prior to first dosing and 3, 7, 17, 21, 31 and 35 days post the first dosing. Serum samples were kept frozen below – 65 ºC for up to 3 months until analysis (Rabbit hs-CRP (high-sensitivity C-reactive protein) ELISA Kit, Catalog No. E-EL-RB2267, Elabscience®).
Blood samples for determination of antibody level (immunogenicity phase) were collected in serum separation tubes from all animals assigned to the recovery phase (5 males and 5 females per group), at the following time points: once prior to first dosing, 1 day prior to second and third dosing sessions and prior to scheduled termination (3 weeks post the 3rd dosing session). Serum samples were stored frozen (below – 65 ºC) until analysis. Blood samples for determination of viremia level were collected in sodium citrate tubes from all animals assigned to the recovery phase (5 males and 5 females per group), at the following time points: 2, 7 and 31 days post the first dosing and prior to the scheduled termination (3 weeks post the 3rd dosing session). Whole blood samples were stored at 2-8ºC until analysis.
Urine collection for viruria levels
Urine samples for detection of viruria were collected from each cage under-tray (wrapped in a clean nylon film) following an overnight period, at the following times points: during the study (the night between days): 2–3, 7–8, 30–31 and 48–49 days post the first dosing session. Individual urine samples were collected from five of ten males and five of ten females per group. All urine samples were stored at 2–8 ºC before testing.
Viremia and viruria determination
Samples were prepared as follows: 200 µl of urine or blood sample were added to 150 µl of lysis buffer (LBF, supplied with the extraction kit) and incubated for 20 min at room temperature for complete lysis. Following lysis, RNA extraction was completed using an RNAdvance Viral kit as per manufacturer’s instructions. Real-time RT-PCR was performed using the Sensi-FAST™ Probe Lo-ROX one-step kit. In each reaction, the primers’ final concentration was 600 nM and the probe concentration was 300 nM. Primers and probes were designed using the Primer Express Software (Applied Biosystems), targeting: (1) the native nucleocapsid gene (N) of VSV Indiana strain and (2) the human codon-optimized SARS-CoV-2 spike gene (S). Thermal cycling was performed at 48 °C for 20 min for reverse transcription, followed by 95 °C for 2 min, and then 45 cycles of 94 °C for 15 s, 60 °C for 35 s. Thermal cycling conditions are from the Berlin protocol published in the WHO recommendation for the detection of SARS-CoV-2 (Corman et al. 2020).
The primers and probes used
N gene: forward: TGATCGACTTTGGATTGTCTTCTAA,
reverse: TCTGGTGGATCTGAGCAGAAGAG,
probe: ATATTCTTCCGTCAAAAACCCTGCCTTCCA;
S gene: forward: GAGTGAGTGTGTGCTGGGACAA,
reverse: AAACACTCCCTCCCTTGGAAA,
probe: AGTTTTCCACAGTCTGCCCCTCATGGA.
The probes used were 6-FAM and ZEN/Iowa Black FQ combination. Assay performance was verified using appropriate controls.
Determination of antibody levels
Antigen microarray was used to quantify S2P-specific antibodies in rabbit serum to evaluate vaccine uptake. The microarray, developed by IIBR (non-GLP) (Fisher et al. 2021, 2022), consists of recombinant, purified SARS-CoV-2 pre-fusion stabilized spike (S2P) protein that was spotted (18 spots) on 16 sun-arrays nitrocellulose-coated slides. Slides were pre-blocked to avoid non-specific binding and stored desiccated until use. Rabbit sera were diluted (1:500) and loaded separately on one of 16 sub-arrays. Following incubation, the slides were washed and probed with anti-rabbit IgG reporter antibody coupled to a fluorescent marker. Following an additional wash step, the resulting fluorescent signal was quantified by scan array. The animals were confirmed as being exposed to the vaccine by evaluating anti‐S2P IgG signal to noise (S/N) ratio, where the negative sample consisted of commercial naïve rabbit serum.
Plaque reduction neutralization test (PRNT50)
PRNT50 has been previously described (Yahalom-Ronen et al. 2020). Briefly, all serum samples were heat inactivated (HI) at 56 °C for 30 min, then diluted in twofold serial dilutions (between 1:20 and 1:640) and incubated with 300 PFU/mL of the SARS-CoV-2 original virus (1 h at 37 °C). Vero E6 cells were infected with the virus–serum mixtures and incubated at 37 °C and 5% CO2 for 72 h. Following incubation, the overlay was aspirated, and the cells were fixed and stained with 1 mL/well of crystal violet solution. Plaques were counted and NT50 was calculated using GraphPad Prism 6 software (GraphPad Software Inc. USA).
Virus stocks of the SARS-CoV-2 original virus (GISAID accession EPI_ISL_406862) to be used in the PRNT tests were propagated (four passages) on Vero E6 cells. All virus stocks were titered on Vero E6 cells as previously described (Yahalom-Ronen et al. 2020). Handling and working with the SARS-CoV-2 virus were conducted in a BSL3 facility in accordance with the biosafety guidelines of the Israel Institute for Biological Research (IIBR).
Necropsy, tissue processing and histopathological evaluation
At each termination time point, the respective animals were initially anesthetized by an i.m. injection of a mixture of ketamine and xylazine (into the dorsal muscles) and then euthanized by an i.v. overdose of Na-pentobarbitone. All animals were subjected to thorough examination, including the external surface of the body, all orifices, cranial, thoracic and abdominal cavities and their contents. Any abnormalities or gross pathological changes observed in tissues and/or organs were recorded, accordingly. A full list of organs/tissues (Bregman et al. 2003) were collected from all animals during each necropsy session and fixed in either 10% neutral buffered formalin (approximately, 4% formaldehyde solution) or Davidson’s solution, for at least 48 h fixation prior to their shipment to Alizée Pathology, Inc., a StageBio company for histopathology processing. The adrenals, brain, epididymides, heart, kidneys, liver, lungs, ovaries/testes, pituitary, salivary glands, spleen, uterus with cervix and thymus were weighed wet as soon as possible following their dissection. The thyroid was weighed following its fixation (at least 24 h fixation).
Slide preparation followed by histopathological examinations was confined to animals assigned to the main phase (sacrificed 3 days post the 3rd dosing session). In addition, the injection sites (both muscle and overlying skin), regional iliac lymph node and spleen from all animals assigned to recovery phase (sacrificed 3 weeks post the 3rd dosing session) and full organs/tissues list of one male from the high-dose recovery group (which exhibited clinical sign) were subjected to histological processing and evaluation. Tissues were trimmed, embedded in paraffin, sectioned at approximately 5 µ thickness and stained with hematoxylin and eosin (H&E). Three slides were prepared from each injection area as follows: one in the middle and two additional sections at approximately 3 mm proximal and distal to the mid-section. In addition, three slides were prepared from the skin at the injection site.
Histopathological evaluation was carried out by a board-certified pathologist. Histopathological changes were described and scored using a semi-quantitative method with five grades (0–4), taking into consideration the severity of the changes (Schafer et al. 2018).
Organ/tissue collection for biodistribution
Organ/tissue collection for biodistribution was carried out during each necropsy session and was limited to the following organs: brain, testis/ovary, liver, thymus, heart, lungs, kidney, spleen, mesenteric lymph node, iliac lymph node, skin and subcutaneous tissue at the injection site, thigh muscle at the injection area, bone marrow and colon. Each organ/tissue was sliced to provide a sample size of at least 30 mg. Organ/samples were placed separately and individually in pre-labeled cryoplastic screw-top tubes, frozen in liquid nitrogen (as soon as possible) and then kept on dry ice until transferred to storage below − 65 ºC at the testing facility. RNA was extracted using TRI Reagent Solution by adding two metal beads and 500 µl TRI Reagent Solution, homogenization in 1300 RPM for 3 min in the Geno/Grinder (Geno/Grinder 2010, SPEXSamplePrep), and addition of 500 µl TRI Reagent Solution according to the manufacturer’s instructions (Thermo Fisher Scientific, Cat AM9738, Lot BCCC8200).
Analysis of rabbit tissue samples was carried out by Hy Laboratories Ltd (Rehovot, Israel) by RT-qPCR, using the same “N” and “S” targeting primer probes as described above. An endogenous control to normalize the amount of template was used (rabbit HPRT):
Forward: CCTTGGTCAAGCAGTATAATCC.
Reverse: AGGGCATATCCTACAACAAACT.
Probe: AGTGTTGGATACAGGCCAGACTTTGTT.
The analysis was limited to three males and three females assigned to the control group and high-dose group (main phase). At the second scheduled termination time point (recovery phase), analysis was carried out on three males and three females assigned to the control group and high-dose group as well, and was limited to the iliac lymph node.
Statistical analysis
Statistical evaluations were performed using R (Version 3.2.1) or Microsoft Excel:
MultiComp.Rnw (validated R-Script for statistical evaluation between multiple groups and/or multiple parameters between the 2 groups).
Prior to application of the appropriate statistical method, a normality test was performed for Gaussian distribution (i.e., Shapiro–Wilk normality test; p < 0.01).
For MultiComp.Rnw.
If the normality test was passed by all groups:
An equal-variance test was performed (i.e., Bartlett test; p < 0.01).
If the Bartlett test was passed, one-way ANOVA with Dunnett's post-test was performed.
If the Bartlett test was not passed, Kruskal–Wallis test with Mann–Whitney U test was performed.
If the normality test was not passed by all groups, Kruskal–Wallis test with Mann–Whitney U test was performed.
Results
In-life examinations
No mortality occurred in any of the animals throughout the entire observation period, and no test item treatment-related clinical signs were observed. One high-dose male rabbit bore less weight on its left hind limb, which began a day following the first dosing session and continued until the scheduled termination, 49 days post the first dosing (recovery animal). There was no similar finding on the right hind limb (which was injected at the same occasion, with the same dose and volume). This may be attributed to inadvertent injection of the sciatic nerve which can result in lameness; however, histopathological evaluation performed 49 days post the first dosing revealed no potential treatment-related lesions in the sciatic nerve, brain or spinal cord. No additional clinical signs were evident in any of the animals throughout the entire study period. No local reactions at the injection site area were observed in any of the animals throughout the entire study period. An increase in mean group body weight was evident in all groups throughout the study period and no statistically significant difference was noted in mean group body weight gain at the end of the study period between the vaccine-treated groups vs. the control group.
A statistically significant increase in the mean group body temperature vs. the control group, within the normal body temperature range of rabbits, was noted in both vaccine-treated groups (females) on the first dosing session and the low-dose-treated group (males) a day following the first dosing (Table S3). Body temperature higher than the normal range of rabbits (i.e., ≥ 39.8 °C, according to the US and European pharmacopeias) was measured in both vaccines-treated groups but also in the control group of both sexes on the day of the second injection, Day 14 (40.37 °C, 40.03 °C, and 40.18 °C in low-dose, high-dose and control females; 39.84 °C, 39.71 °C, and 40.14 °C in low-dose, high-dose and control males, respectively) and, therefore, were not considered be to related to the tested vaccine. No such changes were observed on Day 28, following the third injection.
Food consumption values were within the normal range throughout the study period. All animals exhibited normal appearance of blood vessels and the optic disc during indirect ophthalmoscopy examinations. There were no marked differences in urinalysis values of the animals assigned to the treated groups vs. the control group.
Clinical pathology
Mean group values of most parameters in both vaccine-treated groups were comparable to those of the control group, within each sex. A few statistically significant differences were found; however, these findings can be considered incidental since the values were within the normal range of reference values and/or similar to baseline values. A statistically significant increase in mean fibrinogen level was noted in the low-dose males (374.8 mg/dl) vs. controls (275 mg/dl), 31 days post the first dosing. A similar increase was noted in low-dose females (366.8 mg/dl vs. 297 mg/dl in controls) and in high-dose males (375.5 mg/dl vs. 275 mg/dl in controls); however, these were not statistically significant. Elevation in fibrinogen level is expected following vaccine injection and is generally consistent with immune stimulation and inflammation. A significant increase in mean fibrinogen, an acute phase protein, was noted 3 days post-dosing in the high-dose male group (329 mg/dl vs. 275 mg/dl in controls); however, this increase was mainly attributed to the single male which bore less weight on its left hind limb following injection. No similar increases in fibrinogen levels were noted on day 3 in either low-dose males or in low- and high-dose females. In all cases, the fibrinogen level returned to baseline values on 49 days post-first dosing.
C-reactive protein (CRP)
Group mean values of CRP of both the low- and high-dose- treated groups (males and females) were similar to that of the control group at the various time points and were in the range of expected normal values (0.2-2 ng/ml). No dose response was observed at any specific time point, indicating no systemic inflammation response.
Antibody levels
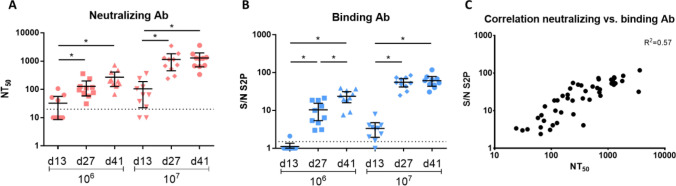
All vaccine-injected rabbits responded to the vaccine by induction of SARS-CoV-2 neutralizing antibodies and spike-specific binding antibodies. A gradual increase in neutralizing and binding antibody titers at both vaccine doses was noted during vaccination (Fig. 1a, b). The evaluated response kinetics of both antibody types was comparable, and a significant increase of around one log was observed from Day 13 to Day 41. A neutralizing response to the primary vaccination was detected (Day 13) in 40% of the animals vaccinated with the low dose and 80% of the animals vaccinated with the high dose. The initial binding response was detected in 10% of the low dose and 90% in the high dose. Following boost (Day 27), all animals responded, as demonstrated by a significant increase in both neutralizing and binding antibodies. The average values of the higher vaccine dose (107) were consistently higher as compared to the lower dose (106) indicating a dose-dependent effect of the immune response. Following the third vaccination, both binding and neutralizing titers increased in the low-dose group, while in the high-dose group the titers reached maximal values following the second vaccination. A high correlation was observed between the binding and neutralizing antibody titers by the linear regression test (p < 0.0001, R2 = 0.57, Fig. 1c). The ratio between the neutralizing and the binding antibodies points to a balanced response with no temporal dominance for either the functional (e.g., naturalizing antibodies) or the binding antibody response. No statistical differences were observed between females and males for both the neutralizing and binding antibodies.
Fig. 1.
Binding and neutralizing antibodies following vaccination with rVSV-ΔG-SARS-CoV-2-S vaccine. Temporal neutralizing (a) and binding (b) antibodies response in rabbits, 13 days following prime vaccination, 13 days following boost (Day 27) and 13 days following second boost (Day 41). Determination of neutralizing antibodies was conducted using the PRNT with SARS-CoV-2. NT50 values are presented. Binding antibodies were determined on S2P antigen. Signal to noise (S/N) values are presented. Dotted line represents the limit of detection of each test. Significance was determined by unpaired t test, *p < 0.05. c Correlation between the neutralizing and binding antibodies following vaccination (Days 13, 27 and 41 post the prime vaccination). Linear regression statistics were performed (p < 0.0001, R2 = 0.57)
Viremia and viruria
Detectable traces of RNA were noted in blood samples in four of five males (< 10 PFU/ml) and in one of five females (< 1 PFU/ml) assigned to the high-dose-treated group, 2 days post the first dosing. Vaccine strain RNA was not detected by 7 days post the first dosing. All other blood samples were free of vaccine strain RNA. No shedding of the viral vaccine was evident, as determined by an absence of vaccine strain RNA in the urine samples collected 2, 7, 31 and 49 days post the first dosing.
Biodistribution
Detectable levels of vaccine RNA were limited to the draining lymph node of the injection site, the iliac lymph node, noted in five samples out of six collected from three males and three females assigned to the high-dose group (main phase), and in five samples out of six collected from three males and three females assigned to the high-dose group (recovery phase). Vaccine RNA was not detected in any other tissues tested (Table S4).
Necropsy and organ weight
No gross pathological findings were observed in any of the animals at the time of their scheduled necropsy excluding hemorrhage-like lesion (dark red color 2 cm × 1 cm) limited to the surface of muscles in one control animal which displayed minimal mixed inflammatory cell infiltration in the histopathological evaluation. The mean group values of most organ weights and organ weight to body weight ratio were comparable to that of control group. A few statistically significant differences in the mean group organ weights and mean group organ weight to body weight ratio between the vaccine-treated groups vs. the control group were noted which can be considered as incidental findings, since no sex or dose dependency was noted and no pathological findings were evident in the histopathological evaluation. A statistically significant increase in lung weight to body weight ratio was noted in both vaccine-treated groups (females) 49 days post the first dosing, related to barbiturate injection for euthanasia (Grieves et al. 2008).
Histopathological evaluation
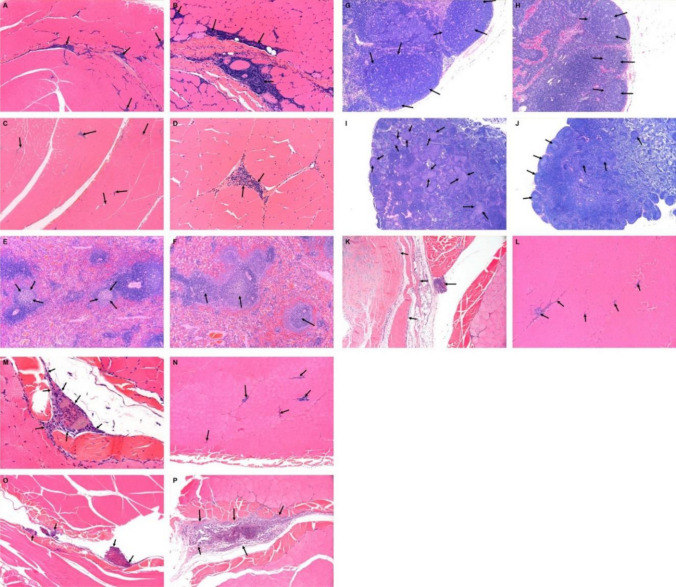
Three days post the last vaccination session (end of main phase), treatment-related histopathological changes were seen in all treated groups, without an apparent dose relation in incidence and/or severity. The changes were seen at the injection sites, iliac and mesenteric lymph nodes, and spleen (Fig. 2).
Fig. 2.
Rabbit histopathology following three repeated i.m. injections with rVSV-ΔG-SARS-CoV-2-S vaccine. Three days post the last vaccination session (magnification is shown in brackets, e.g., (× 10)): a, b injection site (high dose, 107 PFU/animal) arrows indicate mild multifocal mixed mononuclear and polymorphonuclear cell infiltration. The inflammatory reaction is associated with minimal fiber degeneration. c, d Injection site (control animal) arrows indicate minimal multifocal mixed mononuclear and polymorphonuclear cell infiltration. The inflammatory reaction is not associated with fiber degeneration (a, c: × 4; b, d: × 20). e Spleen of high-dose (107 PFU) animal (× 10) and f control animal (× 10): arrows indicate mild germinal center increased cellularity in high-dose-treated animal (e) which was not observed in the control animal (f). g Iliac lymph node (regional lymph node to the injection site) of high-dose (107 PFU) animal (× 10) and (h) of a control animal (× 10): arrows indicate mild germinal center increased cellularity (g) which was not observed in the control animal (h). i Mesenteric lymph node of high-dose (107 PFU) animal (× 4) and (j) of a control animal (× 4): arrows indicate mild germinal center increased cellularity (i) which was not observed in the control animal (j). Three weeks post the last vaccination session (end of recovery period): injection site analysis (k–p): k, p control animal: arrows indicate minimal multifocal mixed mononuclear and polymorphonuclear cell infiltration and minimal fibrosis (k, × 10) and minimal granulomatous inflammation, i.e., foreign body granuloma (p × 10). l–m Low-dose (106 PFU) animal—arrows indicate mild multifocal mixed mononuclear and polymorphonuclear cell infiltration (l × 10) and minimal granulomatous inflammation, i.e., foreign body granuloma (m × 40). n–o High-dose (107 PFU) animal—arrows indicate mild multifocal mixed mononuclear and polymorphonuclear cell infiltration (n × 10) and minimal granulomatous inflammation, i.e., foreign body granuloma (o × 10)
The changes at the injection sites consisted of multifocal minimal myofiber necrosis, multifocal minimal to mild mixed inflammatory cell infiltration (i.e., cells consisting of a mixture of polymorphonuclear cells, lymphocytes and macrophages within the skeletal muscle and/or interstitial fat tissue). This inflammatory reaction was sometimes seen in the surrounding fat tissue, but did not extend into the sciatic nerve itself. This change is considered an extension of the primary injection site inflammation (Fig. 2a–d).
The injection site lesions of minimal focal epidermal crust formation and epidermal hyperplasia, sporadically seen in control and treated animals, as well as multifocal mixed mononuclear cell infiltration seen in the underlying skeletal muscle of the control group, are considered to be related to the needle puncture trauma, as previously reported in similar circumstances (Ramot et al. 2019).
In the iliac and mesenteric lymph nodes, and spleen, minimal to mild germinal centers’ increased lymphocytic cellularity (i.e., follicular hyperplasia) were noted (Fig. 2e–j).
Additional changes observed, such as mononuclear and or inflammatory cell infiltration, seen in the liver and thyroids of control and treated animals are considered to be incidental findings, unrelated to treatment.
Three weeks post the last vaccination session (end of the recovery period), treatment-related histopathological changes were seen in the skeletal muscle of all treated groups, without an apparent dose relation in incidence and/or severity. The changes showed a clear trend for recovery compared to some of the morphological changes observed at the former time point (main phase). No treatment-related changes were seen in the skin (overlying the injection sites), spleen and iliac lymph nodes. The changes at the injection sites consisted of multifocal minimal to mild mixed inflammatory cell infiltration (i.e., cells consisting of a mixture of polymorphonuclear cells, lymphocytes and macrophages within the skeletal muscle and/or interstitial fat tissue). This inflammatory reaction was sometimes seen in the surrounding fat tissue, but did not extend into the sciatic nerve itself (Fig. 2k–p). Compared to the end of the main phase (former time point), this lesion was of relatively comparable incidence and/or severity in the females; however in males, a trend for reduced incidence and/or severity was noted. In addition, minimal focal fibrosis, minimal focal granulomatous inflammation and almost no presence of myofiber necrosis were seen at this time point, all suggesting a trend for progressive recovery. The granulomatous reaction was of foreign body nature, indicative of progressing absorption and elimination of the injected vaccine. There was almost complete healing of the focal skin changes in comparison to the former time point. The observed treatment-related changes at the injection site are judged to be non-adverse, based on the analysis of the findings, taking into consideration the relatively minor severity and expected time-related reversibility. The injection site inflammation may be robust, particularly when formulated with an immune activator (i.e., adjuvant). When unaccompanied by clinical signs (other than transient elevations in body temperature and redness/swelling at the injection site) and with evidence of reversibility (i.e., no evidence of long-term impairment, excessive scarring/fibrosis, progression, etc.), injection site findings, even marked to severe, are generally considered nonadverse (Kerlin et al. 2016; Sellers et al. 2020).
Discussion
In light of the global need for the development of vaccines for SARS-CoV-2, we developed rVSV-ΔG-SARS-CoV-2-S, a clinical stage (Phase 2) replication competent recombinant VSV-∆G-spike vaccine against SARS-CoV-2. In rVSV-ΔG-SARS-CoV-2-S, the G gene which encodes the viral envelope glycoprotein of VSV was deleted from the viral genome and replaced with the S gene of SARS-CoV-2 (Yahalom-Ronen et al. 2020). The recombinant rVSV platform is widely used for vaccine development and was utilized by Merck Sharp & Dohme B.V. for the development of ERVEBO®, for the prevention of Zaire ebolavirus-caused disease (available online https://www.fda.gov/vaccines-blood-biologics/ervebo). ERVEBO®’s proven efficacy and safety profile validates the VSV-ΔG backbone as a platform to produce effective and safe vaccines (Fathi et al. 2019). It is noteworthy that during the development and manufacturing processes, rVSV-ΔG-SARS-CoV-2-S vaccine acquired spontaneous mutations at sites of major importance to antibody-mediated immunity, some of which are identical, or correspond to some of the key mutations or areas of SARS-CoV-2 variants of concern (VOCs), namely N501, E484, Q493 and G685. The ability of rVSV-ΔG-SARS-CoV-2-S to maintain neutralizing antibody response against alpha, gamma and delta variants was recently demonstrated in human sera (Yahalom-Ronen et al. 2022). This may provide an advantage of rVSV-ΔG-SARS-CoV-2-S vaccine against future SARS-CoV-2 VOCs and illustrates the potential value of employing a vector-based vaccination platform.
An effective immune response of vaccine is characterized by the production of neutralizing antibodies, generation of a T-cell response, and avoidance of immune-enhanced disease (i.e., increased disease severity on viral challenge), (Creech et al. 2021). This was clearly demonstrated in a series of nonclinical studies (Madar-Balakirski et al. 2022; Yahalom-Ronen et al. 2020) in which the safety, immunogenicity and efficacy of rVSV-ΔG-SARS-CoV-2-S were evaluated in four animal species (rodent and non-rodent), using multiple doses (up to 108 PFU/animal) and dosing regimens.
Here, results from a GLP study conducted in rabbits are reported in which repeat-dose toxicity, local tolerance, immunogenicity and biodistribution were evaluated. The rabbit, a non-rodent animal species, is considered a suitably robust, sensitive and regulatory acceptable animal species for vaccine testing. Comparison between human and rabbit hACE2 at the 29 amino acid residues of ACE2 interface (that may interact with the SARS-CoV-2 spike glycoprotein RBD) revealed a relatively high degree of conservation (Chan et al. 2020), which further supported the relevance of this model. In addition, data from the preliminary nonclinical safety study demonstrated immunogenicity at clinically relevant doses, as well as histopathological changes in vaccinated rabbits (i.e., increased lymphocytic cellularity in the germinal centers of the spleen and regional lymph node) were considered secondary changes that reflect the expected antigenic stimulation in response to the vaccine, further substantiating the use of this model for the GLP toxicity study.
Three repetitive administrations of rVSV-ΔG-SARS-CoV-2-S vaccine were not associated with major local or systemic adverse effects throughout the observation period in both sexes. Body temperature higher than the normal body temperature range of rabbits (i.e., ≥ 39.8, according to the US and European pharmacopeias) was measured in all groups of either sex on Day 14 (second administration). Since these increases in body temperature were also observed in the control animals, they were considered as not related to the vaccine but rather to possible immune response to the human serum albumin component in the formulation buffer. Indeed, rabbits immunized with human serum albumin can respond with fever upon challenge with the antigen and febrile tolerance to human serum albumin can be induced (Mott and Wolff 1966).
Hematology, biochemistry and coagulation parameters were comparable to that of the control group within each sex. A few statistically significant differences were generally considered incidental, since the values were within the normal range of reference values and/or similar to baseline values. Of note, reversible increases in fibrinogen levels were observed 3 days post the third dosing session in both the low- and high-dose-treated animals vs. control. Fibrinogen is a classic acute phase reactant, and its elevation is generally consistent with immune stimulation and inflammation associated with intramuscular delivery. Interestingly, no dose response induction was observed in hs-CRP levels in the test item-treated animals when compared to the control group at any specific time point or when compared to baseline mean values of each group, indicating that no systemic inflammation response was observed.
In the biodistribution analysis, detection of rVSV-ΔG-SARS-CoV-2-S vaccine RNA was limited to the regional iliac lymph node in the main and recovery animals assigned to the high-dose groups, reflecting the capture of the viral vaccine by the host’s immune system. The vaccine was not detected in all other tissues tested. In terms of immunogenicity, a dose-dependent increase in binding and neutralizing antibody titers was observed following the vaccination sessions. The kinetics of the evolving response in both antibody types was comparable and dose dependent and a high correlation was observed between the two antibody types (p < 0.0001, R2 = 0.57). In addition, the observed treatment-related histopathological findings (i.e., lymphoid hyperplasia in local lymph nodes and spleen, and mild local inflammation) were consistent with what could be expected from an immunogenic vaccine with immune stimulation.
Conclusions
In view of the reported findings and under the conditions of this study, it can be concluded that rVSV-ΔG-SARS-CoV-2-S vaccine, following three repeated vaccination sessions by i.m. injections, 2 weeks apart, to male and female NZW rabbits, is not associated with major local or systemic adverse effects, and thus is considered safe. This study was part of the nonclinical package that supported the initiation of clinical trials (NCT04608305), currently in Phase 2.
Supplementary Information
Below is the link to the electronic supplementary material.
Acknowledgements
We acknowledge the IIBR administrative personnel for their commitment to the project. We thank Yossi Shlomovich, Noa Caspi and Ruchama Brody from IIBR animal husbandry. We thank Dr. Liora Madar-Shapiro for supporting the design and interpretation of the biodistribution phase and for valuable comments. We thank Tal Levin-Harrus, Shkedia Kohen, Raphael Lioz, Yael Kdoshim, Oshrit Shaniand Julia Vilenski from Envigo CRS (Israel) Ltd., Ness Ziona, Israel; We thank Dr. Ortal Shimon and Dr. Eyal Mor from Hy Laboratories Ltd. We thank Dr. Tami Horovitz from Gsap, Israel for scientific editing of the manuscript. We thank the Bundeswehr Institute of Microbiology, Munich, Germany, that kindly provided SARS-CoV-2 (GISAID accession EPI_ISL_406862).
Author contributions
Conceived of or the designed study: A.R., M.S., N.K., H.M., N.M.B; performed research: S.M., B.P., E.V., H.T., H.A., L.C., R.A., Y.Y.R., H.L., A.B.D., D.S., A.M., M.F., E.F., S.W., N.K., A.N., N.P., T.I.; contributed new methods or models: S.M., B.P., E.V., H.T., H.A., L.C., R.A., Y.Y.R., H.L., A.B.D., D.S., A.M., M.F., E.F., S.W., N.K., A.N. N.P., T.I.; wrote the paper: A.R., M.S., A.B.D., A.M., N.K., A.N., S.Y., N.P., T.I., H.M., N.M.B.; all authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding. Patents: patent application for the described vaccine was filed by the Israel Institute for Biological Research.
Declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical standards
This study was performed following an application-form review by the National Council for Animal Experimentation and after receiving approval (No. IL-20-6-249) that the study complies with the rules and regulations set forth.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Amir Rosner and Michal Steiner contributed equally to this work. Hadar Marcus and Noa Madar-Balakirski contributed equally to this work.
Contributor Information
Hadar Marcus, Email: hadarm@iibr.gov.il.
Noa Madar-Balakirski, Email: noa.madar@gmail.com.
References
- Bregman CL, Adler RR, Morton DG, Regan KS, Yano BL, Society of Toxicologic P Recommended tissue list for histopathologic examination in repeat-dose toxicity and carcinogenicity studies: a proposal of the Society of Toxicologic Pathology (STP) Toxicol Pathol. 2003;31(2):252–253. doi: 10.1080/01926230390183751. [DOI] [PubMed] [Google Scholar]
- Chan JF, Zhang AJ, Yuan S, et al. Simulation of the clinical and pathological manifestations of coronavirus disease 2019 (COVID-19) in a Golden Syrian Hamster Model: implications for disease pathogenesis and transmissibility. Clin Infect Dis. 2020;71(9):2428–2446. doi: 10.1093/cid/ciaa325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corman VM, Landt O, Kaiser M, et al. Detection of 2019 novel coronavirus (2019-nCoV) by real-time RT-PCR. Euro Surveill. 2020 doi: 10.2807/1560-7917.ES.2020.25.3.2000045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Creech CB, Walker SC, Samuels RJ. SARS-CoV-2 vaccines. JAMA. 2021;325(13):1318–1320. doi: 10.1001/jama.2021.3199. [DOI] [PubMed] [Google Scholar]
- Fathi A, Dahlke C, Addo MM. Recombinant vesicular stomatitis virus vector vaccines for WHO blueprint priority pathogens. Hum Vaccin Immunother. 2019;15(10):2269–2285. doi: 10.1080/21645515.2019.1649532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fisher M, Levy H, Fatelevich E, et al. A serological snapshot of COVID-19 initial stages in Israel by a 6-Plex antigen array. Microbiol Spectr. 2021;9(2):e0087021. doi: 10.1128/Spectrum.00870-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fisher M, Manor A, Abramovitch H, et al. A novel quantitative multi-component serological assay for SARS-CoV-2 vaccine evaluation. Anal Chem. 2022 doi: 10.1021/acs.analchem.1c05264. [DOI] [PubMed] [Google Scholar]
- Grieves JL, Dick EJ, Jr, Schlabritz-Loutsevich NE, et al. Barbiturate euthanasia solution-induced tissue artifact in nonhuman primates. J Med Primatol. 2008;37(3):154–161. doi: 10.1111/j.1600-0684.2007.00271.x. [DOI] [PubMed] [Google Scholar]
- Kerlin R, Bolon B, Burkhardt J, et al. Scientific and regulatory policy committee: recommended ("Best") practices for determining, communicating, and using adverse effect data from nonclinical studies. Toxicol Pathol. 2016;44(2):147–162. doi: 10.1177/0192623315623265. [DOI] [PubMed] [Google Scholar]
- Madar-Balakirski N, Rosner A, Melamed S, et al. Preliminary nonclinical safety and immunogenicity of an rVSV-DeltaG-SARS-CoV-2-S vaccine in mice, hamsters, rabbits and pigs. Arch Toxicol. 2022;96(3):859–875. doi: 10.1007/s00204-021-03214-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mott PD, Wolff SM. The association of fever and antibody response in rabbits immunized with human serum albumin. J Clin Invest. 1966;45(3):372–379. doi: 10.1172/JCI105352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramot Y, Kannan K, Reddy S, Krishnappa H, Dillberger JE, Nyska A. Acute histopathologic findings related to needle puncture trauma during subcutaneous injection in the sprague-dawley rat model. Toxicol Pathol. 2019;47(1):93–96. doi: 10.1177/0192623318808989. [DOI] [PubMed] [Google Scholar]
- Schafer KA, Eighmy J, Fikes JD, et al. Use of severity grades to characterize histopathologic changes. Toxicol Pathol. 2018;46(3):256–265. doi: 10.1177/0192623318761348. [DOI] [PubMed] [Google Scholar]
- Sellers RS, Nelson K, Bennet B, et al. Scientific and regulatory policy committee points to consider*: approaches to the conduct and interpretation of vaccine safety studies for clinical and anatomic pathologists. Toxicol Pathol. 2020;48(2):257–276. doi: 10.1177/0192623319875085. [DOI] [PubMed] [Google Scholar]
- Suder E, Furuyama W, Feldmann H, Marzi A, de Wit E. The vesicular stomatitis virus-based Ebola virus vaccine: From concept to clinical trials. Hum Vaccin Immunother. 2018;14(9):2107–2113. doi: 10.1080/21645515.2018.1473698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yahalom-Ronen Y, Tamir H, Melamed S, et al. A single dose of recombinant VSV-G-spike vaccine provides protection against SARS-CoV-2 challenge. Nat Commun. 2020;11(1):6402. doi: 10.1038/s41467-020-20228-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yahalom-Ronen Y, Erez N, Fisher M, et al. Neutralization of SARS-CoV-2 variants by rVSV-DeltaG-spike-elicited human sera. Vaccines (Basel) 2022 doi: 10.3390/vaccines10020291. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.