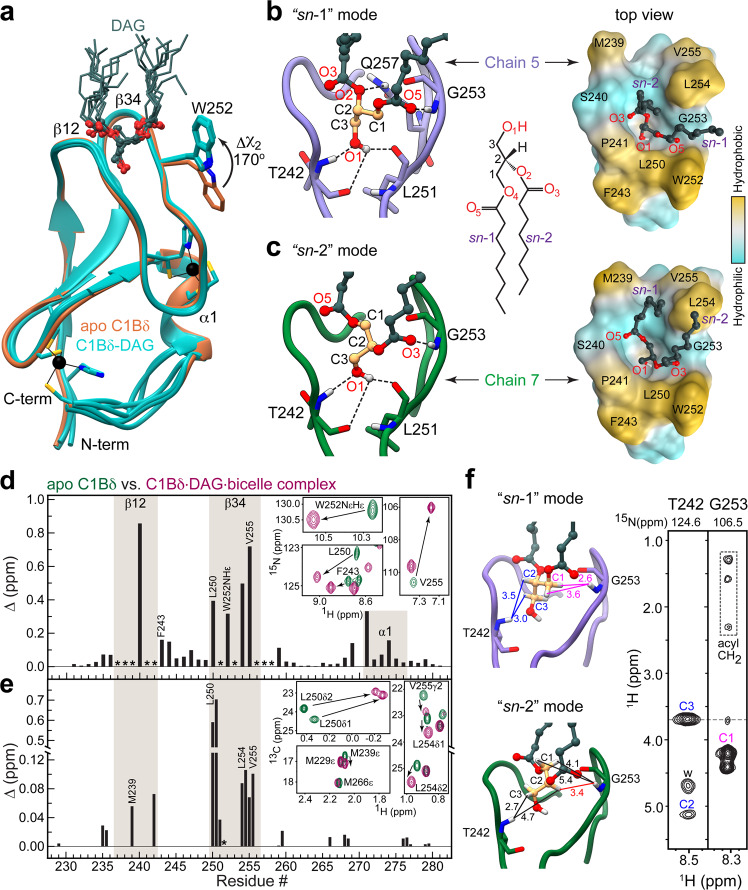
Fig. 2. Stereospecificity of DAG binding by C1Bδ.
a Backbone superposition of 8 DAG-complexed C1Bδ chains of the AU (cyan, PDB ID: 7L92) onto the structure of apo C1Bδ (sienna, PDB ID: 7KND). The sidechain of Trp252 reorients towards the tips of membrane-binding β12 and β34 loops upon DAG binding. DAG adopts one of the two distinct binding modes: “sn-1” (b) or “sn-2” (c). The formation of the C1Bδ-DAG complex in bicelles is reported by the chemical shift perturbations (CSPs) of the amide 15NH (d) and methyl 13CH3 (e) groups of C1Bδ. Asterisks denote residues whose resonances are broadened by chemical exchange in the apo-state. The insets show the response of individual residues to DAG binding through the expansions of the 15N–1H and 13C–1H HSQC spectral overlays of apo and DAG-complexed C1Bδ. f 1H–1HN Thr242 and Gly253 strips from the 3D 15N-edited NOESY-TROSY spectrum of the C1Bδ-DAG-bicelle complex. The protein-to-DAG NOE pattern is consistent with the distances observed in the “sn-1” mode (Chain 5, light purple) but not the “sn-2” mode (Chain 7, green). All distances are in Å and color-coded in the “sn-1” complex to match the labels in the spectrum; “w” denotes water protons. The medium-range NOE that would be characteristic of the “sn-2” complex is shown in red.