Fig. 3. Roles of C1Bδ loops in lipid binding.
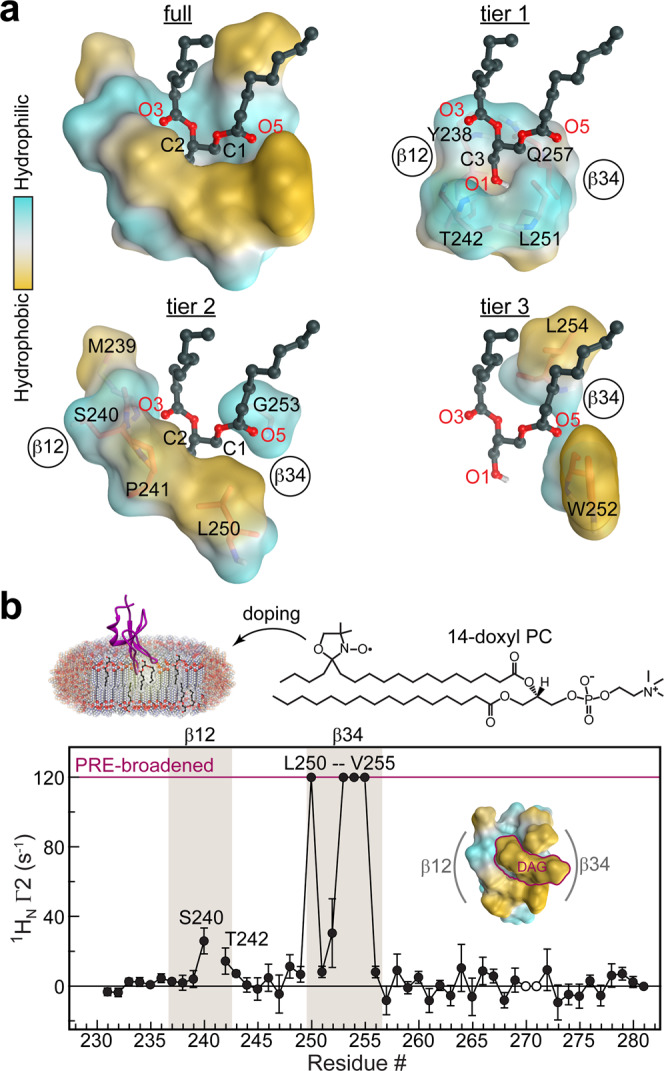
a Polar backbone atoms and hydrophobic sidechains of DAG-interacting C1Bδ residues create a binding site whose properties are tailored to capture the amphiphilic DAG molecule. This is illustrated through the deconstruction of the “sn-1” binding mode into three tiers that accommodate the glycerol backbone (tier 1), the sn-1/2 ester groups (tier 2), and the acyl chain methylenes (tier 3). b Residue-specific lipid-to-protein PRE values of the amide protons, 1H Γ2, indicate that loop β34 is inserted deeper into the membrane than β12. The PRE value for Trp252 is that for the indole NHε group. Cross-peaks broadened beyond detection in paramagnetic bicelles are assigned an arbitrary value of 120 s−1. His270 and Lys271 cross-peaks are exchange-broadened and therefore unsuitable for quantitative analysis (open circles). The PRE values were derived from 1HN transverse relaxation rate constants collected on a single sample in the absence (diamagnetic) and presence (paramagnetic) of 14-doxyl PC. The error was estimated using the r.m.s.d. of the base plane noise. The inset shows the top view of the “sn-1” mode C1Bδ-DAG complex color-coded according to the hydrophobicity.
