Fig. 2.
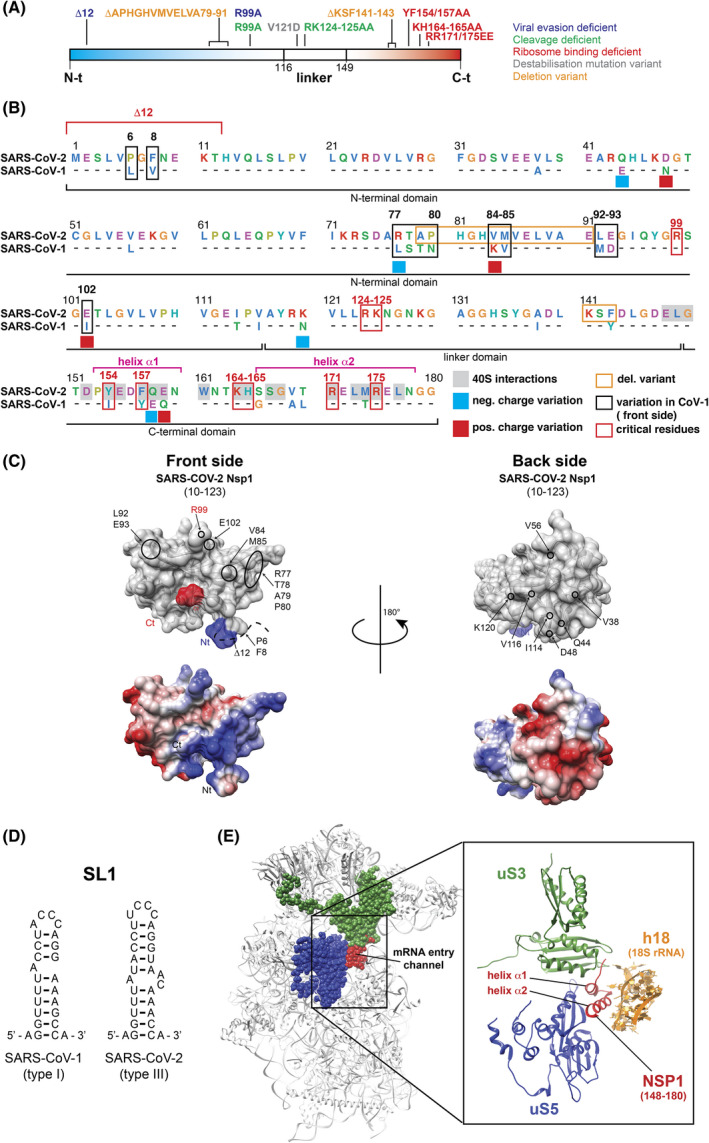
Non‐Structural Protein 1 or NSP1. (A) Linear representation of the three domains of SARS‐CoV‐2 NSP1: the Nt‐domain in blue, the linker domain and the Ct‐domain in red. The mutations of the residues that have been shown to be important for the functions of NSP1 are shown according to the color code indicated on the right. (B) Protein sequence alignment of SARS‐CoV‐2 and SARS‐CoV‐1 NSP1 proteins. For SARS‐CoV‐1, only the divergent amino acids are shown. The NSP1 proteins are subdivided into three domains: the N‐terminal domain, the central linker domain, and a C‐terminal domain. The amino acids are shown according to the following color code: negatively charged amino acids in pink, hydrophobic amino acids in blue, positively charged amino acids in green, aromatic amino acids in cyan, glycines and prolines in orange. Residues involved in interactions with ribosomal components are shaded in gray in SARS‐CoV‐2 [41, 42, 53]. Negative charge variations from SARS‐CoV‐2 to SARS‐CoV‐1 are indicated by blue squares, and positive charge variations are indicated by red squares. Residues that are divergent on the front side of NSP1 are boxed in black. Critical residues implicated in various functions of NSP1 are boxed in red. Deletions that have been found in SARS‐CoV‐2 variants are boxed in orange. (C) Surface representation of crystal structure of SARS‐CoV‐2 NSP1 from residues E10 to L123 (PDB: 7K7P) [50]. The upper panels are two views from the front (left) and back sides (right) of NSP1. The N‐terminal end is shown in in blue and the C‐terminal end in red. The position of residue R99 in SARS‐CoV‐2 NSP1 is indicated in red. Divergent residues from SARS‐CoV‐1 are circled in black. The lower panels represent the electrostatic surfaces of the protein with negative and positive charges colored in red and blue, respectively. (D) Secondary structures of SL1 from SARS‐CoV‐1 (left) and SARS‐CoV‐2 (right). (E) Cryo‐EM structure of the SARS‐CoV‐2 NSP1‐ribosomal 40S complex (PDB: 6ZLW) [41]. The C‐terminal domain of NSP1 is shown in red at the mRNA entry channel. The interactions between NSP1 (red) and the ribosomal proteins uS3 (green), uS5 (dark blue) and helix h18 of the 18S rRNA (orange) are shown.
