FIGURE 1.
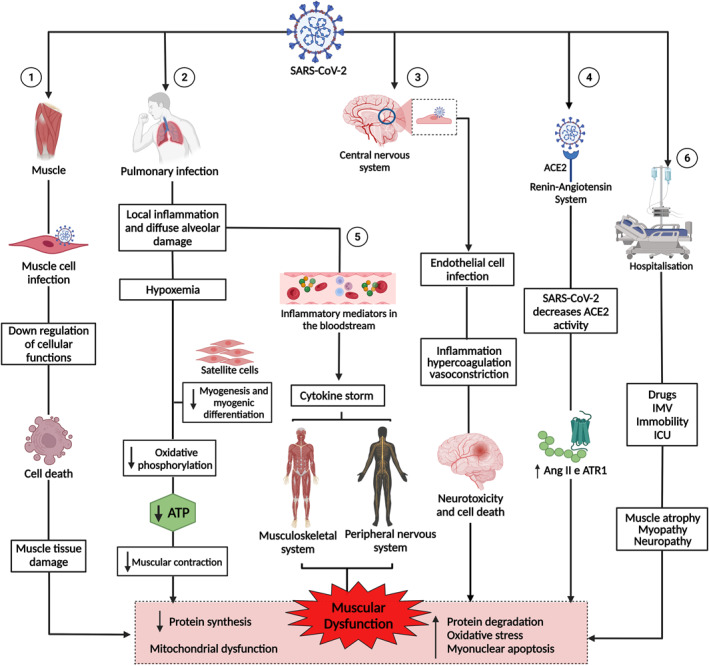
Pathogenesis of muscle dysfunction caused by SARS‐CoV‐2. 1: SARS‐CoV‐2 infects the muscle cell and uses cell machinery for replication, resulting in cell death and tissue damage. 2: SARS‐CoV‐2 infects lung cells, causing a local inflammatory response, diffuse alveolar damage, and hypoxaemia, interfering with myogenesis and myogenic differentiation and muscle metabolism and energy production. 3: SARS‐CoV‐2 infection of endothelial cells results in inflammation, hypercoagulation, and vasoconstriction, leading to neurotoxicity and cell death. 4: The binding of SARS‐CoV‐2 to angiotensin‐converting enzyme 2 (ACE2) negatively regulates the activity of the enzyme, favouring the high expression of angiotensin II (Ang2) and its receptor, angiotensin type 1 receptor (ATR1), leading to muscle atrophy and fibrosis. 5: The exacerbation of inflammation in the lungs increases inflammatory mediators, which are transported by the blood to other organs and systems. In the muscle, inflammatory cytokines increased muscle proteolysis and decreased protein synthesis. In the peripheral nervous system, antibodies attack nerves, causing damage to the axon or myelin. 6: Hospitalisation due to COVID‐19 can cause muscle damage due to the use of drugs and sedatives, as well as mechanical ventilation and immobility. 1, 2, 3, 4, 5, and 6 lead to muscle dysfunction, characterised by decreased synthesis and increased protein degradation, increased oxidative stress, myonuclear apoptosis, and mitochondrial dysfunction. This figure was created with Biorender.com
