Abstract
Immunoglobulin (Ig)A vasculitis/nephropathy is a systemic immune complex‐mediated vasculitis. Although severe acute respiratory syndrome coronavirus 2 (SARS‐CoV‐2) vaccination is widely recommended in individuals without specific allergy to the vaccine components, it is arguable whether vaccination is advisable for patients with IgA vasculitis or for predisposed individuals. We and others have presented cases of IgA vasculitis occurring after SARS‐CoV‐2 vaccination. In total, these 19 cases, including ours, involved predominantly female patients, and half of them were suffering from de novo vasculitis onset. The most frequent manifestation was gross hematuria (89.5%) while skin lesions were relatively infrequent, occurring in only five cases (26.3%), of which three (15.8%) were confirmed to be IgA vasculitis. Taken together, these cases suggest that SARS‐CoV‐2 vaccination might be a trigger for development/deterioration of IgA vasculitis/nephropathy.
Keywords: de novo onset, deterioration, immunoglobulin A nephropathy, immunoglobulin A vasculitis, severe acute respiratory syndrome coronavirus 2 vaccination
1. INTRODUCTION
Immunoglobulin (Ig)A vasculitis, which belongs to the same category of vasculitis as IgA nephropathy, is a systemic immune complex‐mediated vasculitis, characterized by palpable purpura, hematuria, abdominal pain, and arthralgia, frequently followed by upper respiratory tract infection. 1 Recently it has been demonstrated that IgA vasculitis/nephropathy occurs in association with administration of certain drugs and vaccinations. 2 Although severe acute respiratory syndrome coronavirus 2 (SARS‐CoV‐2) vaccination is widely recommended in individuals without specific allergy to the vaccines or their components, 3 it is arguable whether vaccination is suitable for patients with IgA vasculitis or for predisposed individuals. We present a case of IgA vasculitis occurring after SARS‐CoV‐2 vaccination, and we review 18 similar cases previously reported in the literature to discuss the pros and cons of this vaccination in the context of IgA vasculitis.
2. CASE REPORT
A 16‐year‐old girl visited us for purpuric rash on both legs for 9 days. She did not have any history of diseases except for hay fever and intermittent abdominal pain since the age of 11 years. She received the first dose of the Pfizer‐BioNTech BNT16B2b2 mRNA vaccine, reporting mild fatigue and myalgias with low‐grade fever lasting 2 days post‐injection. She subsequently noticed pin‐head‐sized papules on both legs and had recurrent epigastric pains of 5–10 min duration. Pregnancy test was negative. Physical examination disclosed multiple 1–3‐mm palpable purpura (Figure 1) and a slight left knee joint swelling. Laboratory data revealed proteinuria, hematuria, and a mild elevated level of immune complex (4.0 μL/mL; normal, <3.0). Platelet count, coagulation profile, fibrinogen, quantitative immunoglobulins (Ig), including IgA/G/M, electrolytes, kidney, and liver function, were all within normal levels. Histology of the skin lesion on her right leg demonstrated that lymphocytes and neutrophils infiltrated densely around vessels in the upper dermis with nuclear dusts (Figure 2a). Granular deposition of IgA and IgM was found with direct immunofluorescent staining (Figure 2b). A diagnosis of IgA vasculitis was made. She was admitted for bedrest and p.o. administration of diphenyl sulfone. Both rash and arthralgia resolved within a week. Abdominal pain gradually alleviated; however, hematuria persisted for several months. Thereafter, the patient gave written informed consent to the publication of her case details.
FIGURE 1.
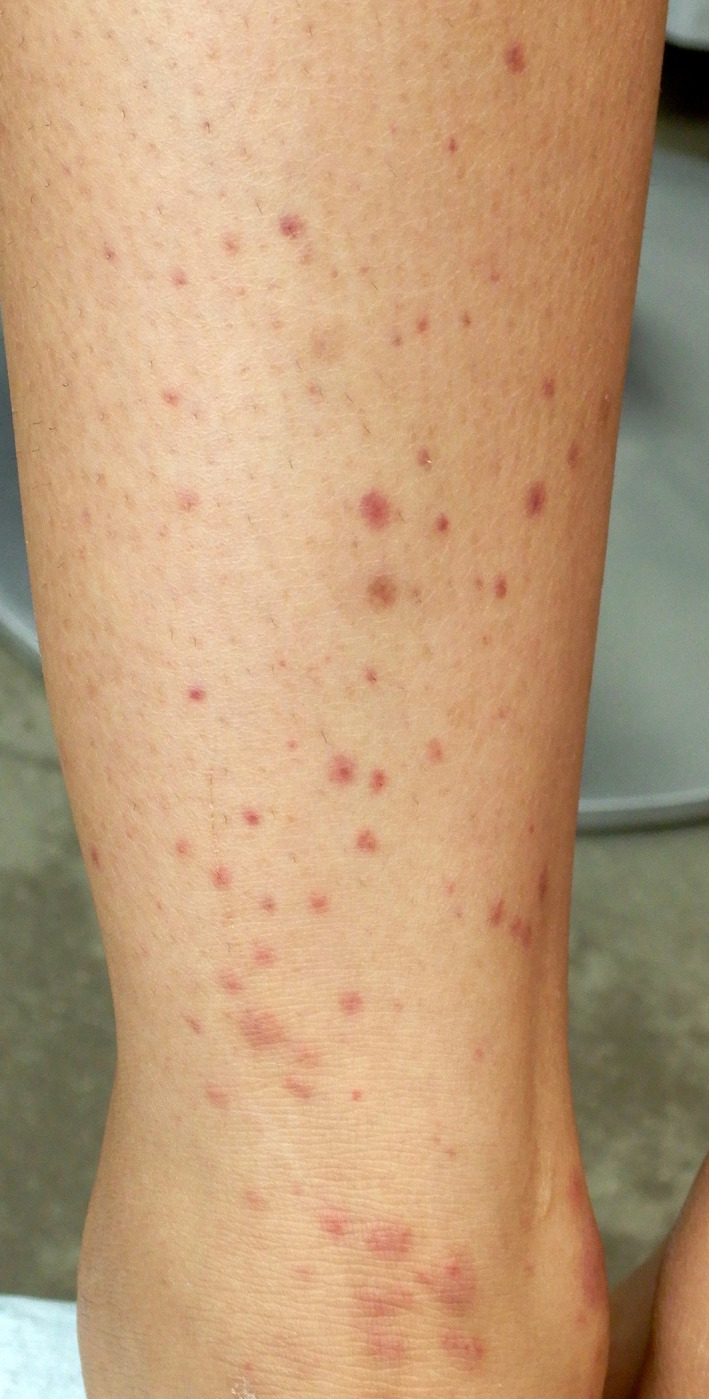
Palpable purpura of legs
FIGURE 2.
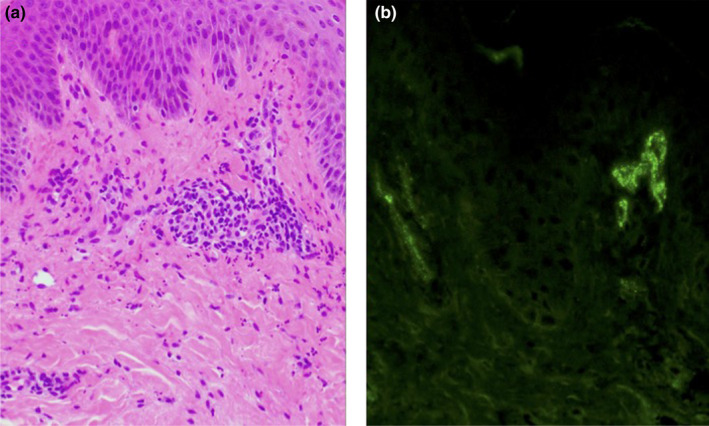
Histological and immunofluorescent features of skin lesions of the right leg. (a) Hematoxylin–eosin staining (original magnification × 200). (b) Fluorescein isothiocyanate‐conjugated anti‐immunoglobulin A antibody (× 200)
3. DISCUSSION
Eighteen previously documented cases of IgA nephropathy/vasculitis occurring post‐SARS‐CoV‐2 vaccination are summarized in Table 1, 4 , 5 , 6 , 7 , 8 , 9 , 10 , 11 , 12 , 13 , 14 along with the present case.
TABLE 1.
Reported cases of immunoglobulin A vasculitis/nephropathy
| Case | Age (years) | Sex | Vaccination | Onset | Symptoms | Ref. | ||||
|---|---|---|---|---|---|---|---|---|---|---|
| Pharm | Dose | Interval (day) | Hematuria | Skin | Others | |||||
| 1 | 38 | F | Mo | 2nd | 1 | Ex (16y) | ++ | − | ND | 8 |
| 2 | 38 | F | Mo | 2nd | 1 | Ex (2y) | ++ | − | ND | |
| 3 | 52 | F | P | 2nd | 1 | Ex (4y) | ++ | − | ND | 4 |
| 4 | 22 | M | Mo | 1st/2nd | 2 | Ex (2y) | ++ | + | Abd, J | 5 |
| 5 | 41 | F | P | 2nd | 2 | Ex (16y) | ++ | − | ND | |
| 6 | 27 | F | P | 2nd | 2 | Ex (1y) | ++ | − | ND | |
| 7 | 22 | F | Mo | 2nd | 2 | Ex (ND) | + | − | ND | 6 |
| 8 | 39 | F | Mo | 2nd | 2 | De novo | + | − | ND | |
| 9 | 50 | M | Mo | 2nd | 1 | De novo | + | − | ND | |
| 10 | 67 | M | Mo | 1st | 30 | De novo | + | + | ND | |
| 11 | 78 | F | Mo | 1st | 7 | Ex (2y) | + | + | Abd | 7 |
| 12 | 42 | F | P | 1st | 4 | De novo | − | + | ND | 12 |
| 13 | 50 | F | Mo | 2nd | 2 | De novo | + | − | ND | 9 |
| 14 | 19 | F | Mo | 2nd | 2 | De novo | ++ | − | ND | |
| 15 | 41 | F | P | 2nd | 1 | Ex (ND) | ++ | − | ND | 14 |
| 16 | 60 | F | P | 2nd | 1 | Ex (ND) | ++ | − | ND | |
| 17 | 40 | F | P | 2nd | 12 | De novo | + | − | ND | 10 |
| 18 | 72 | M | Ox | 1st | 15 | De novo | − | − | ND | 13 |
| 19 | 16 | F | P | 1st | 2 | De novo | + | + | Abd, J | Present case |
| Statistic data | Mean 42.8 | (F/M) 15/4 | (1st /2nd) 6/14 | Mean 4.7 | (D/Ex) 9/10 | 17/19 | 5/19 | 3/19 | ||
Note: Interval, interval between the vaccination and onset. Symptoms: ++, gross hematuria; +, presence of hematuria; −, none; Skin: +, presence; −, no skin lesions. Dose, number of vaccine doses received at time of onset. Numbers of parentheses in onset, period (years) between previous and vaccination‐associated onsets. Statistical data indicates mean values or number of cases in categories.
Abbreviations: Abd, abdominal pain; De novo, de novo onset; Ex, exacerbation of pre‐existing disease; F, female; J, joint pain; M, male; Mo, Moderna; ND, not described; Ox, Oxford‐AstraZeneca; P, Pfizer; Pharm, pharmaceutical company.
Sufferers were predominantly female adults (female/male ratio, 3.75; mean age, 42.8 ± 17.7 years). Although IgA vasculitis is common in children, the age distribution observed might reflect the fact that this vaccination was predominantly given to adults.
The order of frequency in which vaccines induced vasculitis was Moderna (10 cases), Pfizer (eight cases), and Oxford‐AstraZeneca (one case). Twelve cases (63%) occurred after the second injection. The interval between injection and onset ranged 1–30 days (mean, 4.7 ± 7.2 days) but the most frequent interval was 1–2 days.
Approximately half of the cases (47.4%) presented with de novo onset, and the remainder were exacerbated cases of pre‐existing disease. The most frequent manifestation was gross hematuria (17/19 [89.5%]) while skin lesions were observed in only five cases (26.3%), among which three (15.8%) were proven to be IgA vasculitis.
In nine of the 19 cases (47.4%), the disease resolved spontaneously in 2–7 days, while 10 cases (52.6%) required intensive treatments including angiotensin‐converting enzyme inhibitor (one case) 6 and diphenyl sulfone (this case); steroid pulse therapy (two cases); 14 oral corticosteroid administration (four cases); 6 , 7 , 13 renal transplantation (one case) 5 and hemodialysis (one case) 5 .
In our case, the clinical and laboratory features at the first visit fulfilled all the EULAR/PRINTO/PRES criteria for IgA vasculitis. 1 Upon interview, the patient disclosed that she had been suffering intermittent abdominal pains for more than 5 years, which might represent a prodrome of IgA vasculitis. SARS‐CoV‐2 infection reportedly exacerbates IgA vasculitis/nephropathy; 11 however, the pros and cons of SARS‐CoV‐2 vaccination in patients with IgA vasculitis/nephropathy or predisposed individuals remain controversial. 3 , 4 , 5 , 7 , 9 , 10 , 12 , 13 , 14 Despite the uncertainty of pre‐existing disease in this case, the narrow time window between vaccination and disease onset implied a close connection, which prompted us to survey similar cases in the literature.
We found at least 19 cases in the literature, including this case, spanning just 1 year, which seemed highly incidental. Upon closer investigation, in most cases the affected patients were female adults and the interval between SARS‐CoV‐2 vaccination and disease onset was 1–2 days. Interestingly, this contrasts with the cases of SARS‐CoV‐2 infection‐associated IgA vasculitis/nephropathy, which shows male predominance, 11 although the reason for this difference remains unclear. Half of the reported post‐vaccination cases represented deterioration of pre‐existing disease while the remainder were de novo onset cases. Although the appearance of symptoms after vaccination in our case suggested that this was a case of de novo onset, we could not exclude the possibility that the patient was already affected by IgA vasculitis. We should also be aware that approximately half of the cases required intensive treatments including systemic administration of corticosteroids, hemodialysis, and renal transplantation. It is therefore vital that we do not underestimate the adverse effects of vaccines in such patients. It would be better to monitor individuals with a history of IgA vasculitis carefully after SARS‐CoV‐2 vaccination for early detection of deterioration.
In summary, we have presented one case and reviewed 18 incidences of new‐onset/deteriorating IgA nephropathy/vasculitis after SARS‐CoV‐2 vaccination. Clinicians should be aware of SARS‐CoV‐2 vaccination as a possible trigger for development of this disease in patients and predisposed individuals.
CONFLICT OF INTEREST
None declared.
ACKNOWLEDGMENTS
This work was supported in part by grants from the Ministry of Education, Culture, Sports, Science, and Technology (24390276) and the Health and Labor Sciences Research Grants from the Ministry of Health, Labor, and Welfare of Japan.
Hashizume, HP , Ajima, S , Ishikawa, Y . Immunoglobulin A vasculitis post‐severe acute respiratory syndrome coronavirus 2 vaccination and review of reported cases. J Dermatol. 2022;49:560–563. 10.1111/1346-8138.16326
REFERENCES
- 1. Ozen S, Pistorio A, Iusan SM, Bakkaloglu A, Herlin T, Brik R, et al. EULAR/PRINTO/PRES criteria for Henoch‐Schönlein purpura,childhood polyarteritis nodosa, childhood Wegener granulomatosis and childhood Takayasu arteritis: Ankara 2008. Part II: final classification criteria. Ann Rheum Dis. 2010;69:798–806 [DOI] [PubMed] [Google Scholar]
- 2. Rasmussen C, Tisseyre M, Garon‐Czmil J, Atzenhoffer M, Guillevin L, Salem JE, et al. Drug‐induced IgA vasculitis in children and adults: revisiting drug causality using a dual pharmacovigilance‐based approach. Autoimmun Rev. 2021;20:102707 [DOI] [PubMed] [Google Scholar]
- 3. Tanno LK, Berard F, Beaudoin E, Didier A, Demoly P. SARS‐CoV‐2 vaccination and anaphylaxis: recommendations of the French Allergy Community and the Montpellier World Health Organization Collaborating Center. Vaccines (Basel). 2021;9:560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Rahim SEG, Lin JT, Wang JC. A case of gross hematuria and IgA nephropathy flare‐up following SARS‐CoV‐2 vaccination. Kidney Int. 2021;100:238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Perrin P, Bassand X, Benotmane I, Bouvier N. Gross hematuria following SARS‐CoV‐2 vaccination in patients with IgA nephropathy. Kidney Int. 2021;100:466–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Park K, Miyake S, Tai C, Tseng M, Andeen NK, Kung VL. Letter regarding: "a case of gross hematuria and IgA nephropathy flare‐up following SARS‐CoV‐2 vaccination". Kidney Int Rep. 2021;6:2246–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Obeid M, Fenwick C, Pantaleo G. Reactivation of IgA vasculitis after COVID‐19 vaccination. Lancet Rheumatol. 2021;3:e617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Negrea L, Rovin BH. Gross hematuria following vaccination for severe acute respiratory syndrome coronavirus 2 in 2 patients with IgA nephropathy. Kidney Int. 2021;99:1487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Kudose S, Friedmann P, Albajrami O, D'Agati VD. Histologic correlates of gross hematuria following Moderna COVID‐19 vaccine in patients with IgA nephropathy. Kidney Int. 2021;100:468–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Hines AM, Murphy N, Mullin C, Barillas J, Barrientos JC. Henoch‐Schönlein purpura presenting post COVID‐19 vaccination. Vaccine. 2021;39:4571–2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Farooq H, Aemaz Ur Rehman M, Asmar A, Asif S, Mushtaq A, Qureshi MA. The pathogenesis of COVID‐19‐induced IgA nephropathy and IgA vasculitis: a systematic review. J Taibah Univ Med Sci. 2021;17:1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Erler A, Fiedler J, Koch A, Schütz A, Heldmann F. A case of leukocytoclastic vasculitis after vaccination with a SARS‐CoV2‐vaccine ‐ a case report. Arthritis Rheumatol. 2021;73:2188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Badier L, Toledano A, Porel T, Dumond S, Jouglen J, Sailler L, et al. IgA vasculitis in adult patient following vaccination by ChadOx1 nCoV‐19. Autoimmun Rev. 2021;20:102951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Tan HZ, Tan RY, Choo JCJ, Lim CC, Tan CS, Loh AHL, et al. Is COVID‐19 vaccination unmasking glomerulonephritis? Kidney Int. 2021;100:469–71. [DOI] [PMC free article] [PubMed] [Google Scholar]


