Abstract
Aim
Emergency room admissions have decreased globally during the COVID‐19 pandemic, particularly for respiratory diseases. We evaluated hospital admissions for respiratory diseases in the first year of the Italian pandemic and compared them with the corresponding period in 2016–2017.
Methods
The study was carried out at the Sapienza University in Rome, Italy, and covered 9 March to 28 February 2020–2021 and 2016–2017. We tested 85 hospitalised children who were negative for the virus that causes COVID‐19 in 2020–2021 and compared them with 476 hospitalised children from 2016–2017, as we had also tested nasal washing samples for 14 respiratory viruses during that period.
Results
Hospitalisations for acute respiratory tract infections were 82.2% lower in 2020–2021 than 2016–2017. The respiratory syncytial virus (RSV) and several other viruses were detected less frequently during the pandemic. An extraordinary finding was that rhinoviruses remained seasonal. In 2020–2021, we detected a virus in 54.1% of the hospitalised children: rhinoviruses in 41, RSV in 4 and other viruses in 1. This was significantly lower than the 71.6% in 2016–2017: RSV in 130, rhinoviruses in 128 and other viruses in 83.
Conclusion
Pandemic measures dramatically reduced childhood respiratory infections, particularly RSV, but were less effective at reducing rhinoviruses.
Keywords: acute communicable diseases, face masks, hospitalisation, respiratory viruses, social distancing
Abbreviations
- PCR
polymerase chain reaction
- RSV
respiratory syncytial virus
- RT‐PCTR
reverse transcriptase polymerase chain reaction
- SARS‐CoV‐2
severe acute respiratory syndrome coronavirus 2
Key Notes.
We compared respiratory viruses in 85 children who were hospitalised for respiratory diseases during the first year of the Italian pandemic, but were COVID‐19 free, with 476 children from the same period in 2016–2017.
Hospitalisations decreased by 82.2% from 2016–2017 to 2020–2021, and children with viruses fell from 71.6% to 54.5%.
Pandemic restrictions greatly reduced respiratory syncytial infections, but an extraordinary finding was that rhinoviruses remained seasonal.
1. INTRODUCTION
Viral infectious diseases can have severe outcomes in humans, particularly respiratory infections. The major viral respiratory pathogens include influenza viruses, the respiratory syncytial virus (RSV), rhinoviruses, endemic coronaviruses and adenoviruses. For example, there are more than 150 million new cases of bronchiolitis every year worldwide, with an increasing trend in the number of medical visits. 1 , 2 The scientific community has never faced a larger threat of novel emerging viruses, together with the changing behaviour of long‐standing viruses. The discovery of the severe acute respiratory syndrome coronavirus in 2002 and the Middle East respiratory syndrome coronavirus in 2012 highlight how behavioural changes, travel, trade and climate change can contribute to viral outbreaks. Infections from both of these coronaviruses were very low in children, with less than 50 cases reported in the literature and a milder clinical presentation than in adults. 3
The novel severe acute respiratory syndrome coronavirus 2 (SARS‐CoV‐2) has spread rapidly worldwide, raising major global concerns. 4 It was declared a pandemic on 11 March 2020 by the World Health Organization (WHO). Evidence has since confirmed that the SARS‐CoV‐2 infection is less frequent and less severe in children than adults. 5 A number of preventive strategies have been adopted by the Italian Government to contain the SARS‐CoV‐2 pandemic, and this started with a strict lockdown from 9 March 2020. Residents were expected to wear masks from 6 years of age and simple hygiene measures, such as hand washing, were implemented. Mass closures followed, including all schools, factories, pubs, shopping malls and restaurants. Supermarkets remained open, providing daily necessities. Workers were encouraged to work from home if they could. People could only break the quarantine rules in exceptional circumstances. These preventive measures were relaxed on 3 May 2020. Factories, shopping malls, pubs and restaurants reopened, but all schools remained closed. People were allowed to leave their home, their region, visit their parents and take summer holidays. On 14 September 2020, schools reopened in the Lazio region, where this study took place, and children resumed normal activities. Face masks and hand washing were put in place in schools in an effort to prevent the spread of SARS‐CoV‐2.
Our previous study of 15 hospitals in 8 Italian regions reported an average decrease of 81% in paediatric emergency room admissions from the 9 March to 3 May 2020 lockdown period. There was also an 86% decrease in respiratory diseases. 6 We hypothesised that social distancing and simple hygiene measures had drastically reduced the circulation of communicable diseases in the air. Data from the northern hemisphere showed a significant decrease in respiratory infections in children after the lockdown. 7 , 8 , 9 , 10 , 11 , 12 , 13 , 14 Interestingly, reports suggested that the spread of SARS‐CoV‐2 in adults did not affect trends in viral infections from 1 February to 12 April 2020 in Italy. 15 Furthermore, data suggested that rhinoviruses had not been stopped by the preventive measures that were put in place. 16
The hypothesis behind this study was that the strategies to prevent the SARS‐CoV‐2 virus that causes COVID‐19 may have changed the epidemiology of existing respiratory viruses and may have influenced the clinical severity of respiratory diseases in children. A 1‐year surveillance study was established to assess respiratory viral infections in children hospitalised at the Sapienza University of Rome in Italy. Our aim was to evaluate 14 respiratory viruses from the beginning of the lockdown period, namely 9 March 2020 to 28 February 2021, and then compare that data with the corresponding period in 2016–2017. We chose those particular years because we had collected nasal washing samples for each child admitted with acute respiratory tract infections to our hospital during that period. They had been tested for the same 14 respiratory viruses that we tested for in 2020–2021, which enabled us to compare both study periods.
2. MATERIALS AND METHODS
We consecutively enrolled children aged 0–18 (mean age 1.7 ± 0.4 years) who were admitted to the Department of Maternal, Infantile and Urological Sciences of the Sapienza University in Rome, Italy, from 9 March 2020 to 28 February 2021, with acute respiratory tract infections. These included bronchiolitis, asthmatic bronchitis and pneumonia. We retrospectively analysed data from the corresponding period in 2016–2017 and compared the total number of hospitalisations for respiratory diseases and viral aetiology in the pandemic and earlier control group.
The ethics committee of Policlinico Umberto I approved the study (Prot. 107/12). Informed consent was obtained from the infants' parents prior to collecting the clinical and anamnestic data and the nasal washing samples. Specific approval for that data to be included in this study was not required.
The children underwent nasal washing within 24 h of hospitalisation, and all the samples were delivered to the virology laboratory, on ice, within 1–2 h. A panel of nested polymerase chain reaction (PCR) and reverse transcriptase PCR (RT‐PCR) assays was developed in order to detect 14 respiratory viruses. These comprised RSV, influenza viruses A and B, coronaviruses OC43, 229E, NL‐63 and HUK1, adenoviruses, rhinoviruses, parainfluenza viruses 1–3, bocavirus and metapneumovirus. 17 During the 2020–2021 pandemic season, children were only enrolled if their RT‐PCR test was negative for SARS‐CoV‐2. 18
2.1. Study periods
We analysed the differences in the study outcomes during three different time periods in 2020–2021. The first was the strict lockdown period from 9 March to 3 May 2020. The second was from 4 May to 13 September 2020, when the restrictions were more relaxed, but the schools were still closed. The third was from 14 September 2020 to 28 February 2021, when the restriction was still more relaxed and the schools were open. The children were divided into four groups, based on their viral aetiology. The three viral groups were as follows: RSV, rhinoviruses and other viruses, comprising influenza viruses A and B, coronaviruses hCoV OC43, 229E, NL‐63 and HUK1, adenoviruses, parainfluenza viruses 1–3 1–3, bocaviruses and metapneumoviruses. The fourth was a virus‐negative group.
We used SPSS version 25 (IBM Corp) to analyse the data. The chi‐squared test was used to compare the hospitalisations related to acute respiratory tract infections and the viral aetiology between the 2020–2021 and 2016–2017 time periods.
3. RESULTS
When we compared the pandemic and pre‐pandemic periods, we saw a dramatic 82.2% reduction in total hospitalisations for acute respiratory tract infections, from 476 in 2016–2017 to 85 in 2020–2021. RSV and other viruses were detected infrequently in 2020–2021. In contrast, rhinovirus‐related admissions were lower in the spring months of March to May 2020, but then they peaked in the autumn and winter months of September to December 2020. They then showed a second peak in February 2021 (Figure 1). When we compared the data for the circulation of respiratory viruses, these were drastically reduced during the strict lockdown from March to early May 2020 and the more relaxed period from May to August 2020 when some restrictions were still in place, but schools were still closed. Once schools reopened, but some restrictions remained, the only viruses that were isolated by the tests were rhinoviruses (Figure 1).
FIGURE 1.
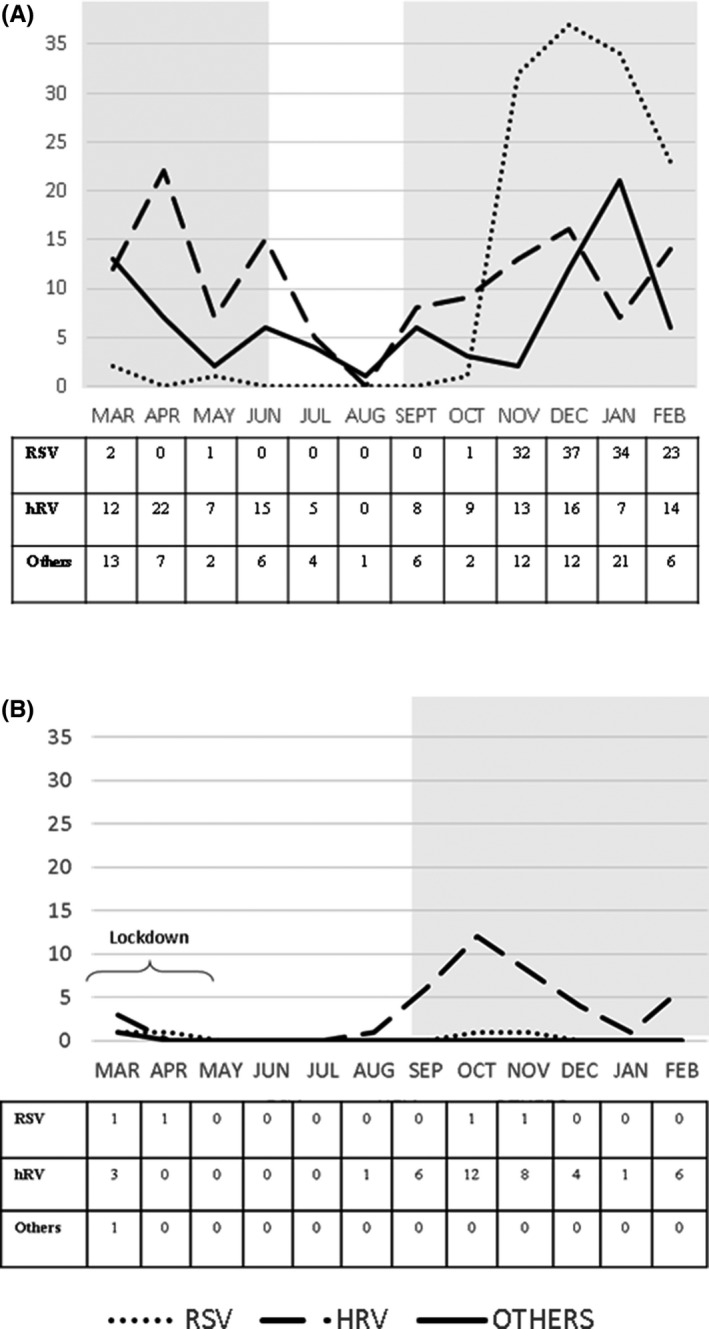
Distribution of viral infections from 9 March to 28 February, 2016–2017 (pre‐pandemic) and 2020–2021 (pandemic). The white area indicates when schools were closed and the grey area indicates when schools were open. The strict lockdown period lasted from 9 March to 3 May 2020, and this was followed by 2 periods of more relaxed restrictions. (A) 2016–2017. Schools were opened until June 2016 and then from September 2016 until the end of the observational period. (B) 2020–2021. Schools were closed during the strict lockdown period and summer and reopened from September 2020 until the end of the observational period
In 2020–2021, we detected a virus in 46/85 (54.1%) hospitalised children: rhinovirus in 41 (48.2%), RSV in 4 (4.7%) and other viruses in 1 (1.2%). Rhinovirus‐related admissions were less frequent from March to May 2020, but showed a first peak from September to December 2020 and a second peak in February 2021 (Figure 1). The cases of RSV were detected in March, April, October and November 2020, and 1 bocavirus was detected in March 2020 (Figure 1).
Our retrospective review of 2016–2017 showed that viruses were isolated in 341/476 (71.6%) hospitalised children: RSVs in 130 (38.1%), rhinoviruses in 128 (37.5%) and other viruses in 83 (24.4%). RSVs were detected in two patients in March 2016, 1 in May 2016 and 1 in October 2016, but the peak in the infection rate was from November 2016 to February 2017, when the other 126 RSVs were recorded. Rhinoviruses were present throughout the observational period, with the exception of August 2016. The other viruses' category comprised influenza viruses in 28 children, the bocavirus in 24, hCoV in 16, metapneumovirus in 13 and the adenovirus in 2. These were detected throughout the year, showing a peak from December 2016 to February 2017 (Figure 1).
4. DISCUSSION
During this 1‐year surveillance study, which started at the beginning of the strict lockdown in Italy, on 9 March 2020 and ran until 28 February 2021, we evaluated 14 respiratory viruses in children hospitalised at the Sapienza University Hospital in Rome, Italy. We found a dramatic reduction in the detection of respiratory viruses when we compared that pandemic period to the same period in 2016–2017. Our results echoed the findings of other studies. 19 , 20 There was a particular reduction in the number of RSVs, which almost disappeared in 2020–2021. However, rhinoviruses were the exception to that rule and continued to infect children during this period.
The COVID‐19 pandemic, and the lockdowns and social distancing strategies that have aimed to contain the virus have changed people's behaviour to a great extent. SARS‐CoV‐2 spreads in exactly the same way as other respiratory viruses, namely through droplets when an infected person coughs, sneezes or talks. The preventive measures that have been applied worldwide have achieved their goal. However, they have also changed the status quo, as they have had a collateral effect on other respiratory viruses. Whenever a novel acute respiratory infection is identified, the WHO recommends the highest available level of infection control precautions, even before the nature of the virus and mode of transmission has been clarified. These include the enhanced hygiene measures, social distancing and face masks that led to the decrease in emergency room admissions, hospitalisations and, in particular, the reductions in respiratory diseases during lockdown periods. 7 It is worth noting that severe respiratory infections, and bronchiolitis epidemics in particular, were at a historical low during the second wave of COVID‐19 in Italy, from November to March. The same pattern was seen in other countries. 21
An extraordinary result of our study was that when we tested for 14 viruses, we found that rhinoviruses were the most frequently detected in hospitalised children during the 1‐year surveillance period from lockdown on 9 March 2020 to 28 February 2021. Rhinoviruses are small naked viruses, which measure 27–30 nm and are members of the Enterovirus genus of the Picornaviridae family. They are the major causes of common colds, and their role in causing lower respiratory tract infections has also been recognised. 22 The reappearance of rhinoviruses in adults in September 2021 coincided with schools reopening, which was consistent with children being a major reservoir for rhinovirus infections. 21 , 23 , 24 We do not know why rhinoviruses have the capacity to overcome respiratory hygiene measures, but several features that are unique to rhinoviruses may play a role in this. First of all, the absence of an outer lipid envelope makes rhinoviruses less vulnerable to both soap and lipophilic sanitisers. Secondly, the icosahedral virions in rhinoviruses are compact and can survive drying, 25 which means that they are more stable on surfaces. 26 Moreover, rhinoviruses particle shedding has mainly been detected in aerosols with a smaller diameter than 4–5 µm. In contrast, influenza viruses, endemic coronaviruses and SARS‐CoV‐2 are enveloped virus particles with spherical or filamentous shapes, which are approximately 100 nm in diameter. They are mainly found in aerosolised respiratory fluid droplets that are >5 µm in diameter. 27 Unfortunately, research has shown that surgical masks are unable to effectively reduce the emission of rhinovirus particles into the environment, either as respiratory droplets or as aerosols. 28 In addition, the most common manifestation of rhinovirus infections is the common cold. A large number of children who have no symptoms, or just a few symptoms, are expected to attend school and can spread the virus. 16 It is interesting to note that from May to August 2020, when preventive strategies were relaxed but schools remained closed, we did not register any rhinovirus peak. This was in contrast with the 2016–2017 period. We can speculate that the relatively low efficacy of surgical masks in the general population, combined with the reduced social distancing that was frequently seen in schools, allowed rhinoviruses to spread. On the contrary, it has been suggested that rhinovirus infections may produce a protective effect against SARS‐CoV‐2 infections, possibly through viral interference. This could have altered the course of the ongoing COVID‐19 pandemic. 16 This hypothesis has been supported by two studies that reported that rhinovirus infections blocked influenza virus infections by stimulating an antiviral defence mechanism in the airway mucosa. 29 , 30
This study had some limitations. Firstly, this was a single‐centre study. We compared respiratory viral infections in children hospitalised in 2020–2021 with those hospitalised in 2016–2017, when a similar surveillance study was performed. However, we do not have similar data about viral aetiology during other epidemic seasons. In addition, children were supposed to wear face masks during school hours from 6 years of age, but this was not directly monitored. Finally, we evaluated several preventive measures simultaneously, so it is difficult to draw inferences about the efficacy of the individual measures.
5. CONCLUSION
This study demonstrated a remarkable beneficial effect of the COVID‐19 preventive measures on child health. Hospitalisations for acute respiratory tract infections were 82.2% lower in 2020–2021 than 2016–2017, and RSVs and several other viruses were detected less frequently during the pandemic. An extraordinary finding was that rhinoviruses remained seasonal. Based on these findings, we suggest that a substantial number of viral infections were prevented. This information should be used to reinforce preventive measures, particularly those related to at‐risk children.
CONFLICT OF INTEREST
The authors have no conflicts of interest to declare.
Nenna R, Matera L, Pierangeli A, et al. First COVID‐19 lockdown resulted in most respiratory viruses disappearing among hospitalised children, with the exception of rhinoviruses. Acta Paediatr. 2022;111:1399–1403. doi: 10.1111/apa.16326
REFERENCES
- 1. Shi T, McAllister DA, O'Brien KL, et al. Global, regional, and national disease burden estimates of acute lower respiratory infections due to respiratory syncytial virus in young children in 2015: a systematic review and modelling study. Lancet. 2017;390(10098):946‐958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Cangiano G, Nenna R, Frassanito A, et al. Bronchiolitis: analysis of 10 consecutive epidemic seasons. Pediatr Pulmonol. 2016;51(12):1330‐1335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Zimmermann P, Curtis N. Coronavirus infections in children including COVID‐19: an overview of the epidemiology, clinical features, diagnosis, treatment and prevention options in children. Pediatr Infect Dis J. 2020;39(5):355‐368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Li T, Tang X, Wu C, et al. The use of SARS‐CoV‐2‐related coronaviruses from bats and pangolins to polarize mutations in SARS‐Cov‐2. Sci China Life Sci. 2020;63(10):1608‐1611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Cristiani L, Mancino E, Matera L, et al. Will children reveal their secret? The coronavirus dilemma. Eur Respir J. 2020;55(4):2000749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Matera L, Nenna R, Rizzo V, et al. SARS‐CoV‐2 pandemic impact on pediatric emergency rooms: a multicenter study. Int J Environ Res Public Health. 2020;17(23):8753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Obando‐Pacheco P, Justicia‐Grande AJ, Rivero‐Calle I, et al. Respiratory syncytial virus seasonality: a global overview. J Infect Dis. 2018;217(9):1356‐1364. [DOI] [PubMed] [Google Scholar]
- 8. Angoulvant F, Ouldali N, Yang DD, et al. Coronavirus disease 2019 pandemic: impact caused by school closure and national lockdown on pediatric visits and admissions for viral and nonviral infections‐a time series analysis. Clin Infect Dis. 2021;72(2):319‐322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Kuitunen I, Artama M, Mäkelä L, Backman K, Heiskanen‐Kosma T, Renko M. Effect of social distancing due to the COVID‐19 pandemic on the incidence of viral respiratory tract infections in children in finland during early 2020. Pediatr Infect Dis J. 2020;39(12):e423‐e427. [DOI] [PubMed] [Google Scholar]
- 10. Nolen LD, Seeman S, Bruden D, et al. Impact of social distancing and travel restrictions on non‐coronavirus disease 2019 (non‐COVID‐19) respiratory hospital admissions in young children in rural Alaska. Clin Infect Dis. 2021;72(12):2196‐2198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Oster Y, Michael‐Gayego A, Rivkin M, Levinson L, Wolf DG, Nir‐Paz R. Decreased prevalence rate of respiratory pathogens in hospitalized patients during the COVID‐19 pandemic: possible role for public health containment measures? Clin Microbiol Infect. 2020;27(5):811‐812. doi: 10.1016/j.cmi.2020.12.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Lee H, Lee H, Song KH, et al. Impact of public health interventions on seasonal influenza activity during the COVID‐19 outbreak in Korea. Clin Infect Dis. 2021;73(1):e132‐e140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Sakamoto H, Ishikane M, Ueda P. Seasonal influenza activity during the SARS‐CoV‐2 outbreak in Japan. JAMA. 2020;323(19):1969‐1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Yeoh DK, Foley DA, Minney‐Smith CA, et al. Impact of coronavirus disease 2019 public health measures on detections of influenza and respiratory syncytial virus in children during the 2020 Australian winter. Clin Infect Dis. 2021;72(12):2199‐2202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Sberna G, Amendola A, Valli MB, et al. Trend of respiratory pathogens during the COVID‐19 epidemic. J Clin Virol. 2020;129:104470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Jones N. How COVID‐19 is changing the cold and flu season. Nature. 2020;588(7838):388‐390. [DOI] [PubMed] [Google Scholar]
- 17. Pierangeli A, Scagnolari C, Trombetti S, et al. Human bocavirus infection in hospitalized children in Italy. Influenza Other Respir Viruses. 2008;2(5):175‐179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Mancino E, Cristiani L, Pierangeli A, et al. A single centre study of viral community‐acquired pneumonia in children: no evidence of SARS‐CoV‐2 from October 2019 to March 2020. J Clin Virol. 2020;128:104385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Partridge E, McCleery E, Cheema R, et al. Evaluation of seasonal respiratory virus activity before and after the statewide COVID‐19 shelter‐in‐place order in Northern California. JAMA Netw Open. 2021;4(1):e2035281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Park S, Michelow IC, Choe YJ. Shifting patterns of respiratory virus activity following social distancing measures for Coronavirus Disease 2019 in South Korea. J Infect Dis. 2021;224(11):1900‐1906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Guedj R, Lorrot M, Lecarpentier T, Leger PL, Corvol H, Carbajal R. Infant bronchiolitis dramatically reduced during the second French COVID‐19 outbreak. Acta Paediatr. 2021;110(4):1297‐1299. 10.1111/apa.15780 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Papadopoulos NG, Bates PJ, Bardin PG, et al. Rhinoviruses infect the lower airways. J Infect Dis. 2000;181(6):1875‐1884. [DOI] [PubMed] [Google Scholar]
- 23. Poole S, Brendish NJ, Tanner AR, Clark TW. Physical distancing in schools for SARS‐CoV‐2 and the resurgence of rhinovirus. Lancet Respir Med. 2020;8(12):e92‐e93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Fong MW, Leung NHL, Cowling BJ, Wu P. Upper respiratory infections in schools and childcare centers reopening after COVID‐19 Dismissals, Hong Kong. Emerg Infect Dis. 2021;27(5):1525‐1527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Palmenberg AC, Spiro D, Kuzmickas R, et al. Sequencing and analyses of all known human rhinovirus genomes reveal structure and evolution. Science. 2009;324:55‐59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Zulli A, Bakker A, Racharaks R, et al. Occurrence of respiratory viruses on school desks. Am J Infect Control. 2021;49(4):464‐468. [DOI] [PubMed] [Google Scholar]
- 27. Milton DK, Fabian MP, Cowling BJ, Grantham ML, McDevitt JJ. Influenza virus aerosols in human exhaled breath: particle size, culturability, and effect of surgical masks. PLoS Pathog. 2013;9:e1003205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Leung NHL, Chu DKW, Shiu EYC, et al. Respiratory virus shedding in exhaled breath and efficacy of face masks. Nat Med. 2020;26(5):676‐680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Casalegno JS, Ottmann M, Duchamp MB, et al. Rhinoviruses delayed the circulation of the pandemic influenza A (H1N1) 2009 virus in France. Clin Microbiol Infect. 2010;16(4):326‐329. [DOI] [PubMed] [Google Scholar]
- 30. Wu A, Mihaylova VT, Landry ML, Foxman EF. Interference between rhinovirus and influenza A virus: a clinical data analysis and experimental infection study. Lancet Microbe. 2020;1(6):e254‐e262. [DOI] [PMC free article] [PubMed] [Google Scholar]


