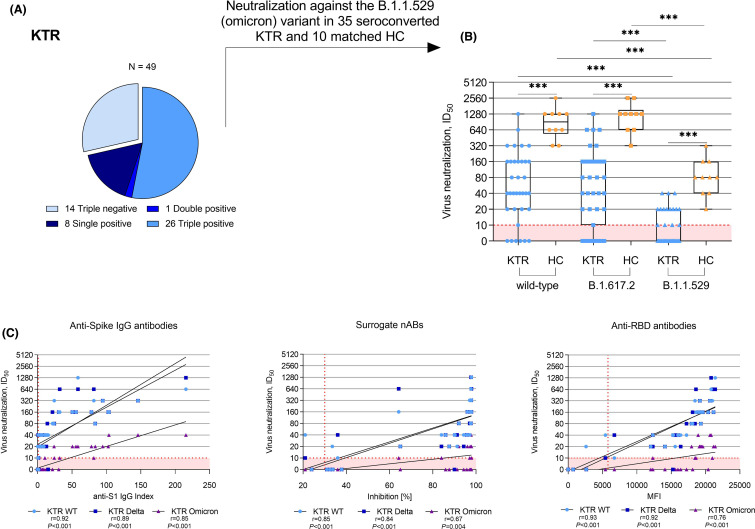
FIGURE 3.
Neutralization of the B.1.1.529 (omicron) variants in kidney transplant recipients showing seroconversion in at least one commercially available assay and healthy controls after a third mRNA vaccine dose. (A) Neutralization of the B.1.1.529 (omicron) variants was assessed in 35 kidney transplant recipients with seropositivity for anti-S1 IgG, surrogate neutralizing, and/or anti-RBD antibodies. (B) Vaccine-induced neutralization of the SARS-CoV-2 wild-type and cross-neutralization of the B.1.617.2 (delta) and the B.1.1.529 (omicron) variants by sera of 35 seroconverted kidney transplant recipients and 10 age- and sex-matched healthy controls after a third mRNA vaccine dose as determined with a live-virus assay. (C) Correlation analyses of anti-spike IgG, surrogate neutralizing, and anti-RBD antibodies with neutralization titers of SARS-CoV-2 wild-type and cross-neutralization of the B.1.617.2 (delta) and the B.1.1.529 (omicron) variants in sera of seroconverted kidney transplant recipients after a third mRNA vaccine dose. The dashed red line indicates the respective cut-off for each assay. HC, healthy controls; ID50, inhibitory dilution 50; KTR, kidney transplant recipients; MFI, mean fluorescence intensity; N, number; RBD, receptor-binding domain; r; Spearman’s rho; snABs, surrogate neutralizing antibodies; WT, wild-type. ***p< .001 [Color figure can be viewed at wileyonlinelibrary.com]