Abstract
The aim of this meta‐analysis is to evaluate the safety of dupilumab use in the management of atopic dermatitis (AD) during the current pandemic regarding the risk and the hazards of COVID‐19 infection. Seven databases (Google Scholar, Web of Science, Scopus, Virtual Health Library, PubMed, System for Information on Gray Literature in Europe, and The New York Academy of Medicine) were searched for eligible studies from inception until November 24, 2021. The quality of evidence was rated using the National Institute of Health and the Joanna Briggs Institute Critical Appraisal tool. Meta‐analysis was performed when the outcome is presented ≥2 studies. A total of 12 papers including 1611 AD patients were included in the study. The prevalence of COVID‐19 in AD treated with dupilumab was 3.2% (95% confidence interval [CI]: 1.7–5.8). COVID‐19 symptoms were reported by five patients who were presented with one or more of the following symptoms (fatigue, loss of taste and smell, runny nose, conjunctivitis, gastrointestinal symptoms, fever, cough, and dyspnea). Only three cases of COVID‐19 were hospitalized with a prevalence of 4.5%, while no patients with COVID‐19 died. Dupilumab is safe regarding the risk and the hazards of COVID‐19 in AD patients. Thus, based on these results continuation of dupilumab in AD patients is recommended, since dupilumab seems to be safe and crucial for a better disease outcome.
Keywords: atopic dermatitis, COVID‐19, dupilumab, meta‐analysis
1. INTRODUCTION
Since the spread of SARS‐CoV‐2 (COVID‐19) pandemic, many concerns arose in dermatology clinics; especially those related to the use of immunosuppressive and immunomodulator treatments for the management of chronic skin disorders. Due to the fear of their possible effect in the context of more severe and symptomatic COVID‐19 infection among patients treated with immunomodulatory drugs, and due to the paucity of the available data to guide clinical recommendations, many patients discontinued their treatment with biologic therapies. 1 , 2 In an Italian cross‐sectional study recruiting patients affected with plaque psoriasis, atopic dermatitis (AD), and hidradenitis suppurativa, 80% of the patients were worried about the hazards of COVID‐19. Though, approximately one fifth of the included patients requested modification or discontinuation of their biologic therapy due to the fear of contracting COVID‐19 infection. 3
Raised concerns arose towards the safety of dupilumab therapy in treating pregnant women with AD. Currently, the available data from case report studies indicate a favorable effect of dupilumab in terms of efficacy that was associated with wide safety margin towards maternal and fetal pregnancy outcomes. 4 , 5 , 6 Moreover, the treatment of the elderly group affected with AD is of paramount interest among the dermatologic society due to the challenging treatment‐induced side effects together with the anticipated reduction of the drug efficacy in this group of patients. In the multicenter study of Patruno and colleagues, elderly AD patients (>65 years old) allocated to receive dupilumab had a significant improvement of their condition. However when compared to patients 18–64 years old, no significant differences observed in terms of efficacy data between the two groups. Moreover, the elderly group had more prevalence of adverse events rather than did the youngest group, 23% and 16%, respectively. 7
Research showed that Th1 immune response is of great importance against viral infections. On the other hand, elevated Th2 cytokines were noticed to impair adequate Th1 immune responses, which are the relevant for AD, a disease characterized by elevated Th2 cytokines with an increased susceptibility to various infections. 8 Furthermore, the elevation of Th2 cytokines in serum of COVID‐19 patients was reported repeatedly specially during the cytokine storm. 9
Dupilumab is a fully humanized monoclonal antibody that targets and blocks interleukin‐4 (IL‐4) receptor‐α, which is shared by both IL‐4 and IL‐13. Hence the latter two interleukins have crucial role in the pathogenesis of AD 10 ; in this context dupilumab was the first FDA approved immunomodulatory medication to treat moderate to severe AD. 1 , 9 , 10
IN March 2020 the European Task Force stated that dupilumab does not seem to increase the risk of viral infections and may be considered as a better option than immunosuppressant in the management of AD, 11 many studies conducted later to examine the safety of dupilumab in AD patients during the COVID‐19 pandemic. 9 , 10 , 12 , 13 In addition, a global database analysis ‐for patients who were treated with dupilumab‐ indicated that dupilumab therapy was associated with mild forms of COVID‐19 in 106 cases while only two cases had severe form of COVID‐19 infection. 14
This meta‐analysis was conducted to evaluate the safety of using dupilumab for the management of AD during the current pandemic regarding the risk and the hazards of COVID‐19 infection.
2. METHOD
2.1. Search strategy
We followed the guidelines of Liberati et al recommendations (PRISMA checklist) for conducting this systematic review and meta‐analysis. 15 From inception until November 24, 2021, a literature search was performed by using the search term “(COVID‐19 OR COVID 19 OR novel coronavirus OR SARS‐CoV‐2) AND (atopic dermatitis) AND (dupilumab)” in seven databases named: Google Scholar, Web of Science, Scopus, Virtual Health Library, PubMed, System for Information on Gray Literature in Europe, and The New York Academy of Medicine. We transported all references and removed duplicated records using Endnote software. Then, all the remaining records were transferred into an Excel sheet for conducting title and abstract screening. We selected potential included papers for another round of full text screening. All the screening process was done by two reviewers and revised by a third review when necessary. One author conducted two manual search methods through PubMed and Google Scholar for retrieving the missed relevant papers.
Inclusion criteria: Any paper reporting COVID‐19 infection in AD patients receiving dupilumab was included.
Exclusion criteria: Conferences abstracts, unavailable full texts, reviews and papers with unreliable data for extraction were all excluded.
2.2. Data extraction and quality assessment
The process of data extraction was done by one author then another author was incorporated for performing substantial revision. The extracted data composed of study characteristics (Study ID, male prevalence, age, diagnosis of COVID‐19 and study design), and outcomes (Prevalence, symptoms, hospitalization and mortality of COVID‐19).
We rated the quality of evidence according to the study design of the included papers (Supplementary file S1). We used the National Institute of Health tool for observational studies and case series studies. 16 While we used Joanna Briggs Institute Critical Appraisal tool for case report papers. 17
2.3. Statistical analysis
The comprehensive meta‐analysis software version 3 was used for the analysis. To avoid hyperinflation of the results, only papers that had a sample size of more than five patients in each outcome were included in the analyses. The pooled prevalence and the corresponding (95% confidence interval [CI]) were used for the analysis. In regards to heterogeneity, we followed the method of Mantel–Haenszel et al, 18 by choosing random model for the presence of heterogeneity otherwise fixed model was chosen. We assessed heterogeneity by p value and I square. 18
3. RESULTS
3.1. Search results and study characteristics
After removing duplicates, 265 records were included. After title and abstract screening, 23 papers were included for further full text review. A total of 10 studies were eligible and finally included in our study. We found additional two papers by manual search trials (Figure 1). 1 , 2 , 9 , 10 , 12 , 13 , 19 , 20 , 21 , 22 , 23 , 24
FIGURE 1.
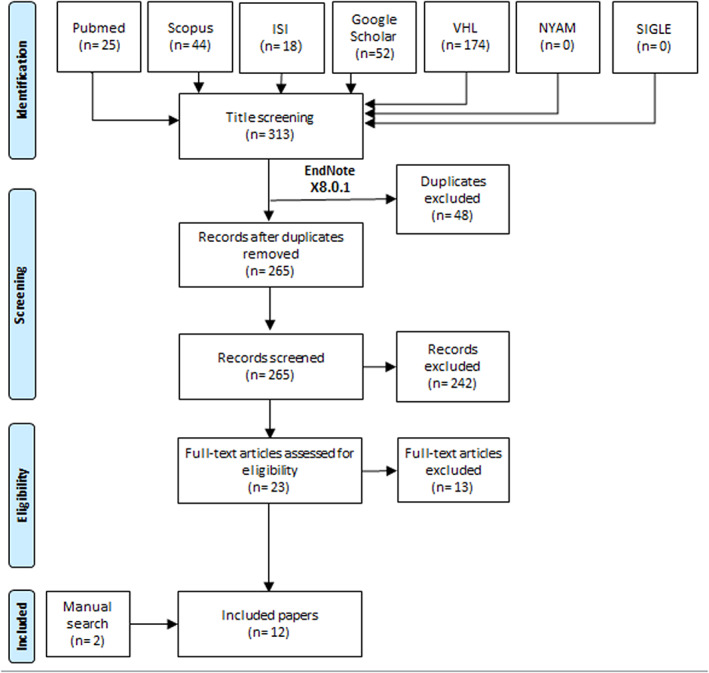
Flow diagram of the study process
Five cross‐sectional studies, two retrospective cohorts, two case series and three case reports (Table 1) with a total sample size of 1611 patients were included. Seven studies were conducted in Italy and the remaining studies were conducted in United States, Colombia, Canada, Poland and Israel. Four studies diagnosed COVID‐19 with polymerase chain reaction (PCR), three studies used PCR and serology testing for diagnosis and five studies did not report the diagnostic method of COVID‐19. A total of 10 studies reported male prevalence that was 51% in our sample of AD patients.
TABLE 1.
Characteristics of the included studies
| Study ID | Study design | Sample size | Age (mean [SD]) | Male prevalence | Diagnosis of COVID‐19 |
|---|---|---|---|---|---|
| Napolitano‐2020‐Italy | Cross‐sectional | 200 | 44 (19.2) | 98 | PCR and serology |
| Georgakopoulos‐2020‐Canada | Retrospective cohort | 162 | NR | NR | NR |
| Hansel‐2021‐Italy | Case series | 9 | 15.7 | 4 | NR |
| Rossi‐2020‐Italy | Cross‐sectional | 71 | 46.5 (18.7) | 41 | NR |
| Stingeni‐2021‐Italy | Case series | 19 | 15.6 | 10 | NR |
| Kridin‐2021‐Israel | Retrospective cohort | 238 | 49.2 (19.9) | 141 | PCR |
| Ungar‐2021‐USA | Cross‐sectional | 632 | 41 (19.1) | 296 | PCR and serology |
| Ferrucci‐2021‐Italy | Cross‐sectional | 245 | NR | NR | NR |
| Carugno‐2020‐Italy | Cross‐sectional | 30 | 35.5 (12) | 20 | PCR and serology |
| Ceryn‐2021‐Poland | Case report | 3 |
27 19 17 |
1 | PCR |
| Caroppo‐2020‐Italy | Case report | 1 | 72 | 1 | PCR |
| Rubiano‐2020‐Colombia | Case report | 1 | 22 | 1 | PCR |
Abbreviations: NR, not reported; PCR, polymerase chain reaction.
3.2. COVID‐19 prevalence
Nine studies reported the prevalence of COVID‐19 infection in AD patients. The prevalence of COVID‐19 was 3.2% (95%CI: 1.7–5.8) using random effect model due to the presence of significant heterogeneity (p < 0.05, I2 = 60) (Figure 2).
FIGURE 2.
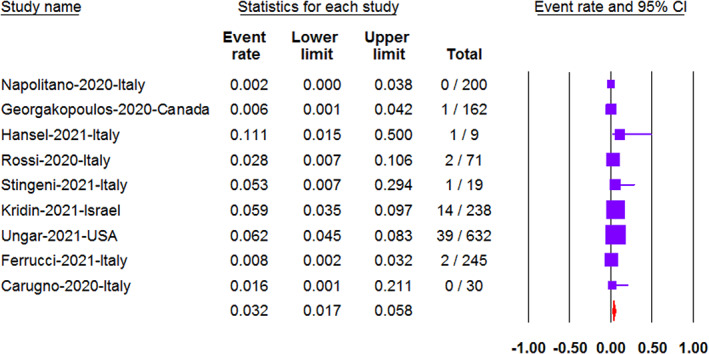
The prevalence of COVID‐19 in AD patients presented with the event rate and the 95% confidence interval (CI)
3.3. COVID‐19 symptoms
COVID‐19 symptoms were reported by two studies including five patients. The five patients were presented with one or more of the following symptoms (fatigue, loss of taste and smell, runny nose, conjunctivitis, gastrointestinal symptoms, fever, cough, and dyspnea) (Table 2).
TABLE 2.
Outcomes of COVID‐19 patients
| Study ID | Study design | Sample size | COVID‐19 symptoms | COVID‐19 outcomes |
|---|---|---|---|---|
| Napolitano‐2020‐Italy | Cross‐sectional | 0 | NA | NA |
| Georgakopoulos‐2020‐Canada | Retrospective cohort | 1 | NR | Recovered |
| Hansel‐2021‐Italy | Case series | 1 | Asymptomatic | Recovered |
| Rossi‐2020‐Italy | Cross‐sectional | 2 | Fever, conjunctivitis and gastrointestinal symptoms | Recovered |
| Fever, cough, and dyspnea | Hospitalized and recovery | |||
| Stingeni‐2021‐Italy | Case series | 1 | Asymptomatic | Recovered |
| Kridin‐2021‐Israel | Retrospective cohort | 14 | NR | All recovered including one hospitalized |
| Ungar‐2021‐USA | Cross‐sectional | 39 | NR | Recovered |
| Ferrucci‐2021‐Italy | Cross‐sectional | 2 | NR | Recovered |
| NR | Hospitalized and recovery | |||
| Carugno‐2020‐Italy | Cross‐sectional | 0 | NA | NA |
| Ceryn‐2021‐Poland | Case report | 3 | Fatigue and loss of taste and smell | Recovered |
| Loss of taste and smell | Recovered | |||
| Fatigue and a runny nose | Recovered | |||
| Caroppo‐2020‐Italy | Case report | 1 | Asymptomatic | Recovered |
| Rubiano‐2020‐Colombia | Case report | 1 | Asymptomatic | Recovered |
Abbreviations: NA, not applicable; NR, not reported.
3.4. COVID‐19 hospitalization
Of the total 65 COVID‐19 patients, only three cases were hospitalized with a prevalence of 4.5%. We could not perform meta‐analysis for hospitalization outcome as the majority of studies had a sample size less than five patients which may progress to results hyperinflation.
3.5. COVID‐19 mortality
All patients of COVID‐19 infection recovered and no patients died.
4. DISCUSSION
Dupilumab, a human monoclonal antibody that binds specifically to the IL‐4Rα subunit of the receptor complexes for IL‐4 and IL‐13 which are core members of Th2 cytokines, 25 is the first FDA approved modality for moderate to severe AD. 26 With the spread of COVID‐19, fearful concerns have been raised against the biologic treatments as general and in the dermatology clinics as well. 27 These concerns were built upon the high burden of COVID‐19 and the subsequent adverse outcomes (symptoms, hospitalization and mortality of COVID‐19) on patients with comorbid illnesses such as diabetes, cancer and other immune‐mediated diseases that had weak immune systems. 28 Moreover, the role of biologic treatment in the immunity modification and suppression raised an alarm towards the risk–benefit ratio towards patients with chronic skin conditions since there are no established guidelines, together with the contradictive evidence upon either continuation or discontinuation. 29
Our systematic review showed that dupilumab did not increase the risk of SARS‐CoV‐2 infection with a prevalence of 3.2% (95%CI: 1.7–5.8) among AD treated patients. The prevalence was similar to patients with psoriasis treated with systemic therapy. 30 Furthermore, the prevalence was higher than that of moderate to severe psoriatic patients who were treated by biologic therapy as 0.2% reported COVID‐19 infection confirmed by nasal swab. 31
COVID‐19 exhibits different symptoms that ranged from mild infection to life‐threatening condition. Fever, cough, fatigue and dyspnea are characteristic features of the disease, however a non‐negligible proportion of COVID‐19 patients presented with asymptomatic infection. 32 In our study, most of AD treated with dupilumab reported asymptomatic infection or mild COVID‐19 symptoms such as fever, cough runny nose and fatigue. And only one patient contracted sever COVID‐19 symptoms that needed hospital admission apart from her elder age (53 years old) and the multiple comorbid conditions (severe obesity, asthma, hypertension and depressive symptoms). 20
In our study no patients with COVID‐19 died due to COVID‐19 itself or any other cause. However, in a worldwide analysis of 10 million COVID‐19 cases from 209 countries, indicated that 2.2%–3.3% of COVID‐19 patients will experience death events. 33 Which indicates the wide safety profile for using dupilumab in the treatment of AD patients whether affected by COVID‐19 or not.
Despite this wide safety margin, the dermatologists questioned about the superiority of dupilumab against other therapies regarding the risk and the hazards of COVID‐19. In a population based study of Kridin and colleagues, the prevalence of COVID‐19 infection in patients treated with dupilumab was higher than those treated with at least 3 months of systemic corticosteroids 5.8% versus 5.1, respectively. However, dupilumab is associated with a reduction in both COVID‐19 hospitalization (0.4% vs. 2%) and mortality events (0% vs. 0.5%) rather than systemic corticosteroids. 10 Furthermore, dupilumb had a lower prevalence of COVID‐19 infection compared to other systemic therapies 6% and 10%, respectively, reported by Unger et al. 9
Currently, we still know a little information about COVID‐19. Till our understanding continues to evolve about the role of dupilumab through decreasing the risk and the adverse outcomes of COVID‐19, immune modification theory plays a substantial role against the cytokine storm that arose from COVID‐19 infection. Dupilumab as an inhibitor for Th2 cytokines—that shown to be elevated in cytokine storm of COVID‐19—may reduce both the incidence and the severity of COVID‐19 symptoms. 9 , 34 , 35
We encountered many limitations in our study. First, there were only two studies comparing dupilumab to other therapies, therefore neither pairwise meta‐analysis or network meta‐analysis were applicable to perform due to the heterogeneity in the compared groups in addition to the low number of studies needed to perform a network meta‐analysis (at least three studies). Therefore, more studies comparing dupilumab to other therapies are needed for the selection of the best therapeutic agent that implies the highest protection against COVID‐19 risk and adverse outcomes. Second, we found a significant heterogeneity across the pooled studies due to the difference in the socioeconomic properties of the included patients as well as the method of COVID‐19 diagnosis. Third, the method of COVID‐19 diagnosis varied across the pooled studies between using PCR, PCR and serology or not reported. Despite that PCR test is the main stay of COVID‐19 diagnosis, the high price for it is affordability in low‐resource settings opened the way for other cheap diagnostic methods such as serology testing. 36
5. CONCLUSION
These findings show that dupilumab is safe regarding the risk and the hazards of COVID‐19 in AD patients. Thus, continuation of dupilumab in AD patients is recommended, since dupilumab seems to be crucial for a better disease outcome.
CONFLICT OF INTEREST
The authors declare no conflicts of interest.
AUTHOR CONTRIBUTION
Amr Ehab El‐Qushayri was responsible for the idea and the study design. All authors shared in screening, extraction, and writing of the full text. All authors approved final version before submission. All steps were supervised by Beatrice Nardone.
Supporting information
Table S1: Quality rating of the prospective cohort studies using National Institute of Health tool.
Table S2: Quality rating of case reports using JBI Critical Appraisal Checklist for Case Reports.
Table S3: Quality rating of the case series study.
El‐Qushayri AE, Mahmoud MA, Salman S, Sarsik S, Nardone B. Dupilumab therapy in atopic dermatitis is safe during COVID‐19 infection era: A systematic review and meta‐analysis of 1611 patients. Dermatologic Therapy. 2022;35(6):e15476. doi: 10.1111/dth.15476
DATA AVAILABILITY STATEMENT
The data that supports the findings of this study are available from the corresponding author upon reasonable request.
REFERENCES
- 1. Ceryn J, Niedźwiedź M, Skibińska M, Ciążyńska M, Lesiak A, Narbutt J. COVID‐19 in patients with atopic dermatitis treated with dupilumab: three cases and a literature review. Clin Cosmet Investig Dermatol. 2021;14:1131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Georgakopoulos JR, Yeung J. Patient‐driven discontinuation of dupilumab during the COVID‐19 pandemic in two academic hospital clinics at the University of Toronto. J Cutan Med Surg. 2020;24(4):422‐423. [DOI] [PubMed] [Google Scholar]
- 3. Bragazzi NL, Riccò M, Pacifico A, et al. COVID‐19 knowledge prevents biologics discontinuation: data from an Italian multicenter survey during RED‐ZONE declaration. Dermatol Ther. 2020;33(4):e13508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Lobo Y, Lee RC, Spelman L. Atopic dermatitis treated safely with dupilumab during pregnancy: a case report and review of the literature. Case Rep Dermatol. 2021;13(2):248‐256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Bosma A, Gerbens L, Middelkamp‐Hup M, Spuls P. Paternal and maternal use of dupilumab in patients with atopic dermatitis: a case series. Clin Exp Dermatol. 2021;46(6):1089‐1092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Akhtar NH, Khosravi‐Hafshejani T, Akhtar D, Dhadwal G, Kanani A. The use of dupilumab in severe atopic dermatitis during pregnancy: a case report. Allergy Asthma Clin Immunol. 2022;18(1):1‐3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Patruno C, Napolitano M, Argenziano G, et al. Dupilumab therapy of atopic dermatitis of the elderly: a multicentre, real‐life study. J Eur Acad Dermatol Venereol. 2021;35(4):958‐964. [DOI] [PubMed] [Google Scholar]
- 8. Wollenberg A, Wetzel S, Burgdorf WH, Haas J. Viral infections in atopic dermatitis: pathogenic aspects and clinical management. J Allergy Clin Immunol. 2003;112(4):667‐674. [DOI] [PubMed] [Google Scholar]
- 9. Ungar B, Glickman JW, Golant A, et al. COVID‐19 symptoms are attenuated in moderate‐to‐severe atopic dermatitis patients treated with dupilumab. J Allergy Clin Immunol Pract. 2021;10(1):134‐142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Kridin K, Schonmann Y, Solomon A, et al. Risk of COVID‐19 and its complications in patients with atopic dermatitis undergoing dupilumab treatment—a population‐based cohort study. Immunol Res. 2021;70(1):106‐113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Wollenberg A, Flohr C, Simon D, et al. European task force on atopic dermatitis statement on severe acute respiratory syndrome coronavirus 2 (SARS‐Cov‐2) infection and atopic dermatitis. J Eur Acad Dermatol Venereol. 2020;34(6):241‐242. [DOI] [PubMed] [Google Scholar]
- 12. Hansel K, Patruno C, Antonelli E, et al. Dupilumab in adolescents with moderate to severe atopic dermatitis: a 32‐week real‐world experience during the COVID‐19 pandemic. Clin Exp Dermatol. 2021;47(1):165‐167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Carugno A, Raponi F, Locatelli A, et al. No evidence of increased risk for COVID‐19 infection in patients treated with dupilumab for atopic dermatitis in a high‐epidemic area‐Bergamo, Lombardy, Italy. J Eur Acad Dermatol Venereol. 2020;34(9):e433‐e434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Mahroum N, Damiani G, Watad A, et al. Higher rates of COVID‐19 but less severe infections reported for patients on dupilumab: a big data analysis of the World Health Organization VigiBase. Eur Rev Med Pharmacol Sci. 2021;25(18):5865‐5870. [DOI] [PubMed] [Google Scholar]
- 15. Liberati A, Altman DG, Tetzlaff J, et al. The PRISMA statement for reporting systematic reviews and meta‐analyses of studies that evaluate health care interventions: explanation and elaboration. PLoS Med. 2009;6(7):e1000100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Health NIo . Quality assessment tool for observational cohort and cross‐sectional studies. National Heart, Lung, and Blood Institute. www.nhlbi.nih.gov/health-pro/guidelines/indevelop/cardiovascular-risk-reduction/tools/cohort Accessed November 5, 2015. 2014.
- 17. Institute JB . JBI critical appraisal checklist for case reports, 2017.
- 18. Mantel N, Haenszel W. Statistical aspects of the analysis of data from retrospective studies of disease. J Natl Cancer Inst. 1959;22(4):719‐748. [PubMed] [Google Scholar]
- 19. Napolitano M, Patruno C, Ruggiero A, Nocerino M, Fabbrocini G. Safety of dupilumab in atopic patients during COVID‐19 outbreak. J Dermatol Treat. 2020;3(1):600‐601. [DOI] [PubMed] [Google Scholar]
- 20. Rossi M, Rovati C, Arisi M, Soglia S, Calzavara‐Pinton P. Management of adult patients with severe atopic dermatitis treated with dupilumab during COVID‐19 pandemic: a single‐center real‐life experience. Dermatol Ther. 2020;33(4):e13765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Stingeni L, Hansel K, Antonelli E, et al. Atopic dermatitis in adolescents: effectiveness and safety of dupilumab in a 16‐week real‐life experience during the COVID‐19 pandemic in Italy. Dermatol Ther. 2021;34(5):e15035. [DOI] [PubMed] [Google Scholar]
- 22. Caroppo F, Biolo G. SARS‐CoV‐2 asymptomatic infection in a patient under treatment with dupilumab. J Eur Acad Dermatol Venereol. 2020;34(8):e368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Ferrucci S, Romagnuolo M, Angileri L, Berti E, Tavecchio S. Safety of dupilumab in severe atopic dermatitis and infection of Covid‐19: two case reports. J Eur Acad Dermatol Venereol. 2020;34(7):303‐304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Ordóñez‐Rubiano MF, Campo I, Casas M. Dupilumab in atopic dermatitis, a protocol for SARS‐COV 2 infected patients. Dermatol Ther. 2020;33(6):e14172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Corren J, Castro M, O'Riordan T, et al. Dupilumab efficacy in patients with uncontrolled, moderate‐to‐severe allergic asthma. The journal of allergy and clinical immunology. In Pract. 2020;8(2):516‐526. [DOI] [PubMed] [Google Scholar]
- 26. Guttman‐Yassky E, Bissonnette R, Ungar B, et al. Dupilumab progressively improves systemic and cutaneous abnormalities in patients with atopic dermatitis. J Allergy Clin Immunol. 2019;143(1):155‐172. [DOI] [PubMed] [Google Scholar]
- 27. Jones ME, Kohn AH, Pourali SP, et al. The use of biologics during the COVID‐19 pandemic. Dermatol Clin. 2021;39(4):545‐553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Sanyaolu A, Okorie C, Marinkovic A, et al. Comorbidity and its impact on patients with COVID‐19. SN Compr Clin Med. 2020;2(8):1069‐1076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Bashyam AM, Feldman SR. Should patients stop their biologic treatment during the COVID‐19 pandemic: Taylor & Francis. 2020;31:317‐318. [DOI] [PubMed] [Google Scholar]
- 30. Baniandrés‐Rodríguez O, Vilar‐Alejo J, Rivera R, et al. Incidence of severe COVID‐19 outcomes in psoriatic patients treated with systemic therapies during the pandemic: a Biobadaderm cohort analysis. J Am Acad Dermatol. 2021;84(2):513‐517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Talamonti M, Galluzzo M, Chiricozzi A, et al. Characteristic of chronic plaque psoriasis patients treated with biologics in Italy during the COVID‐19 pandemic: risk analysis from the PSO‐BIO‐COVID observational study. Expert Opin Biol Ther. 2021;21(2):271‐277. [DOI] [PubMed] [Google Scholar]
- 32. da Rosa MR, Francelino Silva Junior LC, Santos Santana FM, et al. Clinical manifestations of COVID‐19 in the general population: systematic review. Wien Klin Wochenschr. 2021;133(7):377‐382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Cao Y, Hiyoshi A, Montgomery S. COVID‐19 case‐fatality rate and demographic and socioeconomic influencers: worldwide spatial regression analysis based on country‐level data. BMJ Open. 2020;10(11):e043560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Ariëns LF, van der Schaft J, Bakker DS, et al. Dupilumab is very effective in a large cohort of difficult‐to‐treat adult atopic dermatitis patients: first clinical and biomarker results from the BioDay registry. Allergy. 2020;75(1):116‐126. [DOI] [PubMed] [Google Scholar]
- 35. Hamilton JD, Suárez‐Fariñas M, Dhingra N, et al. Dupilumab improves the molecular signature in skin of patients with moderate‐to‐severe atopic dermatitis. J Allergy Clin Immunol. 2014;134(6):1293‐1300. [DOI] [PubMed] [Google Scholar]
- 36. Yusuf L, Appeaning M, Amole TG, et al. Rapid, cheap, and effective COVID‐19 diagnostics for Africa. Diagnostics. 2021;11(11):2105. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1: Quality rating of the prospective cohort studies using National Institute of Health tool.
Table S2: Quality rating of case reports using JBI Critical Appraisal Checklist for Case Reports.
Table S3: Quality rating of the case series study.
Data Availability Statement
The data that supports the findings of this study are available from the corresponding author upon reasonable request.


