Dear Editor,
In an attempt to mitigate the severity of the disease, several medications have been used in patients with COVID‐19 (coronavirus disease 2019). 1 One such therapy is intradermal Mycobacterium w (Mw), which has been used in COVID‐19 patients as an immunomodulatory agent. 1 A randomized trial on 42 patients demonstrated that the use of Mw in addition to standard care resulted in early clinical improvement compared to standard care alone in patients with severe COVID‐19. 2 There were no cutaneous adverse reactions reported in this study. However, we encountered two cases of COVID‐19 that developed local reactions to intradermal injection of Mw and intend to describe these cases so physicians can be aware of such cutaneous reactions.
Both patients described in this report received intradermal Mw (0.3 ml per day: 0.1 ml at each site) at three different sites (both deltoids and lateral aspect of thigh) for 3 consecutive days. A total of nine injections were given to each patient. Patient 1 (56‐year‐old male) developed erythematous painful nodules at all nine injection sites 7 days after administration of the first Mw injection. A few of the nodules were suppurated over the next 3 days leaving behind ulcers (Fig. 1). Patient 2 (48‐year‐old male) developed erythematous painful nodules at multiple sites 6 days after the injections. In this case, the lesions resolved spontaneously without suppuration (Fig. 2). In both cases, lesions were tender and non‐discharging (including ulcers). Local erythema was present, but there were no signs of systemic inflammation. Our experience with intradermal Mw in COVID‐19 patients is limited to the cutaneous adverse effects seen in these patients. Ongoing randomized controlled trials evaluating Mw in critically ill COVID‐19 patients may help shed light on effectiveness and safety. 3
Figure 1.
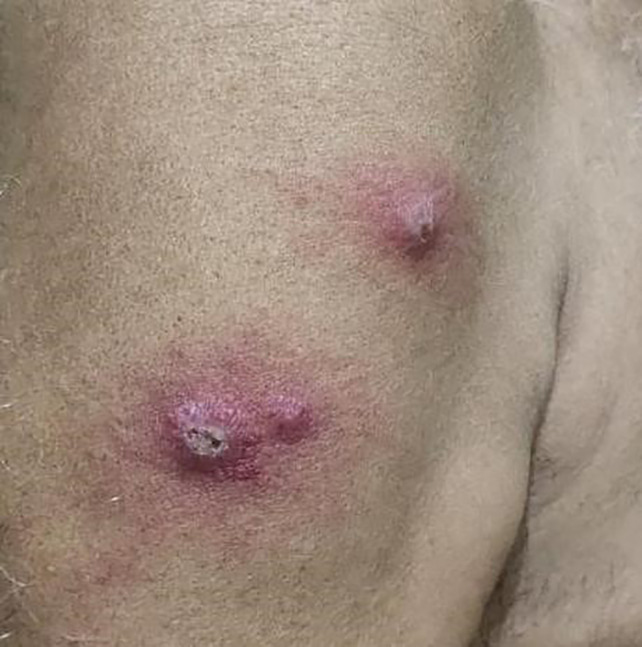
Presence of three erythematous nodules, at Mw injection sites on the right upper arm, with superimposed ulceration in two lesions giving a nodulo‐ulcerative morphology [Colour figure can be viewed at wileyonlinelibrary.com]
Figure 2.
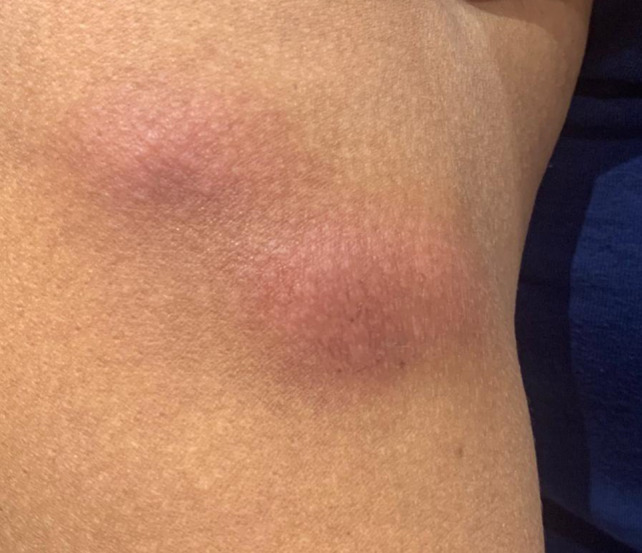
Two grouped erythematous subcutaneous nodules at injection sites on the right arm [Colour figure can be viewed at wileyonlinelibrary.com]
The rationale behind the use of Mw for COVID‐19 is based on its immunomodulatory action. Mw acts through the toll‐like receptors pathway and modulates the T cell responses of the host cells. 2 In support of this, a decrease in inflammatory marker levels was noted in patients with moderate‐to‐severe COVID‐19 who received Mw. 1
Injection site immunological reaction to Mw is a well‐known phenomenon in other conditions, such as leprosy and tuberculosis (TB). 4 , 5 A study among TB patients reported injection site reactions in 82.4% of the patients. 5 The majority (68%) of TB patients experienced mild reactions whereas 12.91% had moderate to severe reactions at the injection site. 5 In a previous study in COVID‐19 patients by Ingale et al., local reactions were observed at the site of injection of Mw in 85.47% of the patients. 3 The majority had mild reactions (54%); patients developed erythema at the site of injection, which was followed by the development of induration and pustule formation. This was followed by the formation of a small punched‐out ulceration which healed spontaneously by scarring. Chawla et al. also reported a similar case where the patient developed injection site ulcerations at all nine injection sites. 6
This report aims to highlight the adverse effects that can arise with the use of this exploratory therapy in COVID‐19 patients. It is important to ask about the history of intradermal injections if one encounters such lesions and recognize that no specific treatment is required as these lesions subside on their own. To conclude, when presented with unusual skin lesions in COVID‐19 patients, physicians should consider COVID‐19 therapy‐related cutaneous reactions apart from the varying COVID‐19 disease‐related skin manifestations that have been described in literature. 7
Acknowledgments
Informed consent was obtained for the use of pictures and reporting as a case report.
Conflict of interest: None.
Funding source: None.
References
- 1. Sehgal IS, Bhalla A, Puri GD, et al. Safety of an immunomodulator Mycobacterium w in COVID‐19. Lung India 2020; 37: 279–281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Sehgal IS, Guleria R, Singh S, et al. A randomised trial of Mycobacterium w in critically ill patients with COVID‐19: ARMY‐1. ERJ Open Res 2021; 7: 00059‐2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. ClinicalTrials.gov . A clinical trial of Mycobacterium w in critically ill COVID 19 patients. Available at: https://clinicaltrials.gov/ct2/show/study/NCT04347174 [accessed October 5, 2021].
- 4. Walia R, Sarathchandra KG, Pandey RM. Field trials on the use of Mycobacterium w vaccine in conjunction with multidrug therapy in leprosy patients for immunotherapeutic and immunoprophylactic purposes. Lepr Rev 1993; 64: 302–311. [DOI] [PubMed] [Google Scholar]
- 5. Sharma SK, Katoch K, Sarin R. Efficacy and safety of Mycobacterium indicus pranii as an adjunct therapy in category II pulmonary tuberculosis in a randomized trial. Sci Rep 2017; 7: 3354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Chawla RK, Chawla AK, Chaudhary G, et al. Mycobacterium W. ‐ an unusual side effect. Indian J Tuberc 2021; 10.1016/j.ijtb.2021.02.013. Epub ahead of print [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Galván Casas C, Català A, Carretero Hernández G, et al. Classification of the cutaneous manifestations of COVID‐19: a rapid prospective nationwide consensus study in Spain with 375 cases. Br J Dermatol 2020; 183: 71–77. [DOI] [PMC free article] [PubMed] [Google Scholar]


